Article Text
Abstract
Objectives To identify whether covid-19 vaccines are associated with menstrual changes in order to address concerns about menstrual cycle disruptions after covid-19 vaccination.
Design Global, retrospective cohort study of prospectively collected data.
Setting International users of the menstrual cycle tracking application, Natural Cycles.
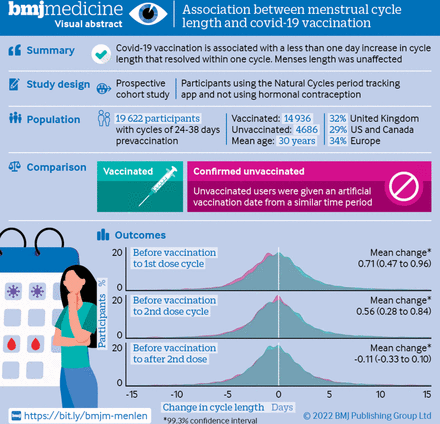
Participants 19 622 individuals aged 18-45 years with cycle lengths of 24-38 days and consecutive data for at least three cycles before and one cycle after covid (vaccinated group; n=14 936), and those with at least four consecutive cycles over a similar time period (unvaccinated group; n=4686).
Main outcome measures The mean change within individuals was assessed by vaccination group for cycle and menses length (mean of three cycles before vaccination to the cycles after first and second dose of vaccine and the subsequent cycle). Mixed effects models were used to estimate the adjusted difference in change in cycle and menses length between the vaccinated and unvaccinated.
Results Most people (n=15 713; 80.08%) were younger than 35 years, from the UK (n=6222; 31.71%), US and Canada (28.59%), or Europe (33.55%). Two thirds (9929 (66.48%) of 14 936) of the vaccinated cohort received the Pfizer-BioNTech (BNT162b2) covid-19 vaccine, 17.46% (n=2608) received Moderna (mRNA-1273), 9.06% (n=1353) received Oxford-AstraZeneca (ChAdOx1 nCoV-19), and 1.89% (n=283) received Johnson & Johnson (Ad26.COV2.S). Individuals who were vaccinated had a less than one day adjusted increase in the length of their first and second vaccine cycles, compared with individuals who were not vaccinated (0.71 day increase (99.3% confidence interval 0.47 to 0.96) for first dose; 0.56 day increase (0.28 to 0.84) for second dose). The adjusted difference was larger in people who received two doses in a cycle (3.70 days increase (2.98 to 4.42)). One cycle after vaccination, cycle length was similar to before the vaccine in individuals who received one dose per cycle (0.02 day change (99.3% confidence interval −0.10 to 0.14), but not yet for individuals who received two doses per cycle (0.85 day change (99.3% confidence interval 0.24 to 1.46)) compared with unvaccinated individuals. Changes in cycle length did not differ by the vaccine’s mechanism of action (mRNA, adenovirus vector, or inactivated virus). Menses length was unaffected by vaccination.
Conclusions Covid-19 vaccination is associated with a small and likely to be temporary change in menstrual cycle length but no change in menses length.
- COVID-19
Data availability statement
No data are available. Data were accessed under a data use agreement with Natural Cycles USA Corp, New York and are not available to third parties.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What is already known on the topic
Menstrual cycle changes after covid-19 vaccination have been reported
Vaccine trials did not prospectively collect outcomes related to menstrual health
A US cohort study of about 4000 individuals (vaccinated and unvaccinated) reported an association between covid-19 vaccines and a slightly longer menstrual cycle of less than one day change from baseline as compared with an unvaccinated group, but no change was noted in length of menses (bleeding)
What this study adds
Evidence of cycle length changes related to covid-19 vaccines in a global cohort of 14 936 vaccinated individuals compared with 4686 unvaccinated individuals
Observed changes were similar across different vaccine types (ie, mRNA, adenovirus vector, or inactivated virus)
Changes were resolved as soon as the next cycle after vaccine receipt
How this study might affect research, practice, or policy
Results can be used to counsel individuals who menstruate about what to expect with covid-19 vaccinations, and underscore the importance of collecting menstrual cycle data during development of future vaccines.
Introduction
A range of menstrual cycle changes after covid-19 vaccination have been reported, including longer and shorter cycles, missed cycles, heavier and lighter menstrual flow, and intermenstrual spotting.1–5 Unfortunately, clinical trials of covid-19 vaccines did not collect outcomes related to menstrual cycles. Official reporting systems like the US Vaccine Adverse Event Reporting System (VAERS) and the UK Medicines and Healthcare Products Regulatory Agency (MHRA)’s Yellow Card surveillance scheme have received reports of menstrual changes after covid-19 vaccination but, as passive systems relying on self-report, the findings are useful for identifying potential issues but are unable to determine prevalence or a clear association. The absence of prospectively collected menstrual cycle data that include an unvaccinated comparison group limits our ability both to address public concerns about the relation between covid-19 vaccination and menstrual cycles and to counsel individuals who menstruate about what to expect with vaccination.
Menstruation is a key patient reported outcome beyond its importance as a general indicator of health and fertility.6 Menstruation is a common, routine bodily function occurring for about a week each month for 40 years: a substantial amount of a person’s lifetime is spent menstruating.7 Any change, even if small and not clinically relevant, is important to the public, and even more so in the context of a new vaccine.8 Although small changes in menstrual characteristics might not be meaningful to clinicians and scientists,9 any perceived effect to a routine bodily function linked to fertility can cause alarm for those experiencing it, and can contribute to vaccine hesitancy. Even small changes, when unanticipated, can have a large adverse impact on the quality of life of people who menstruate and who experience episodes of social embarrassment, anxiety related to uncontained bleeding or fertility planning or prevention, and worry about what bleeding changes mean for their overall health.10–12 The absence of evidence about vaccines and menstrual health coupled with the long standing sex specific research inequities can also be interpreted by the public as a dismissal from the scientific and medical community.13 14
Our previous study was the first to show an association between covid-19 vaccines and menstrual cycle changes.15 However, we included only individuals residing in the US. Now, following the global vaccine rollout, we were able to analysis menstrual cycle tracking data that was prospectively collected using the digital fertility awareness application, Natural Cycles (Natural Cycles USA Corp, NY, US) with an international sample. The purpose of this update is to provide more generalisable results to a broader population and to compare our US findings that covid-19 vaccination is associated with small changes in cycle length during the menstrual cycles when vaccine doses are received and that vaccination is not associated with changes in menses length.
For the visual abstract of this paper, see figure 1.
Visual abstract
Methods
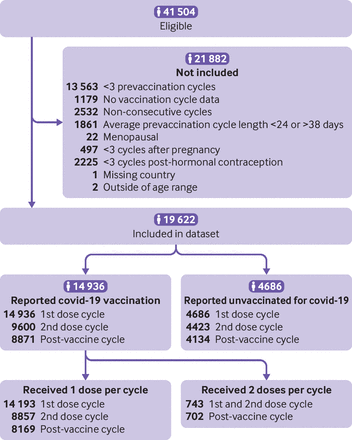
We conducted a retrospective cohort analysis of prospectively collected menstrual cycle data. Menstrual cycle data ranged from 1 October 2020 to 7 November 2021; initial covid-19 vaccine doses were received between 2 January and 31 October 2021. Individuals who use the digital fertility awareness application Natural Cycles voluntarily choose to prospectively track physiological data related to their menstrual cycles for purposes of pregnancy prevention or planning without the use of hormonal methods and consent to the use of their de-identified data for research (consent can be removed, if desired). A detailed description of variables tracked by the app has been published elsewhere.16 To be eligible for study inclusion, individuals had to consent to use of their deidentified data for research purposes, report their covid-19 vaccination status, and record at least one cycle after 1 October 2020. Users were informed of the purpose of the research. We included individuals aged 18-45 years who were at least three cycles after pregnancy or after use of hormonal contraception and not menopausal, with normal prevaccination menstrual cycle lengths (average of 24-38 days),9 and known geographical location. Each individual contributed a minimum of four consecutive cycles of data. For those who received a covid-19 vaccination, we included three prevaccine cycles, at least the first covid-19 vaccine dose cycle and subsequent consecutive cycles recorded in the application through the cycle following the second vaccine dose. For individuals who were not vaccinated, we included four to six consecutive cycles from a similar time period, depending on the number recorded. We excluded individuals with no cycle data for time points after the first covid-19 vaccine dose cycle from analyses for later time points.
Our primary exposure was covid-19 vaccination status, as reported by individuals within the Natural Cycles application. Prompted by in-app messages, individuals recorded their vaccination date or dates or confirmed their unvaccinated status. For a sensitivity analysis focused on vaccine type, we categorised vaccine brands by mechanism of action: mRNA (Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273)), adenovirus vector (Oxford-AstraZeneca (ChAdOx1 nCoV-19), Covishield (ChAdOx1-S), Johnson & Johnson (Ad26.COV2.S), and Sputnik V (Gam-COVID-Vac)), and inactivated virus (Covaxin (BBV152), Sinopharm (BBIBP-CorV), and Sinovac (CoronaVac)).
Our primary outcome was the mean change within individuals in cycle length (in days), from the three cycle prevaccination average to the first vaccine dose cycle, comparing vaccinated and unvaccinated groups. For vaccinated individuals, cycle four was the first vaccine dose cycle; the cycle of the second dose varied based on when this second vaccine dose occurred (cycles four through 13), if applicable. We designated the cycle after the second vaccine dose cycle as the postvaccine cycle in order to determine whether any menstrual cycle changes attenuate or disappear after vaccination. For the unvaccinated cohort, we designated cycle four as the notional first vaccine dose cycle, cycle five (the cycle when the largest proportion of vaccinated individuals received their second dose) as the notional second vaccine dose cycle, and cycle six as the postvaccine cycle; cycles one, two, and three were considered the equivalent of prevaccination cycles.
Secondary outcomes were the same mean change within individuals in cycle length for the second vaccine dose cycle as well as the postvaccine cycle, and corresponding changes in menses length for both doses. For individuals who were vaccinated during their menses, we used the menses length of the vaccine cycle, and for those who were vaccinated after completing their menses, we used the menses length from the subsequent cycle. We also examined the proportion of individuals who had a clinically significant change in cycle length (≥eight days).9
We included additional sociodemographic information collected within the Natural Cycles application via an in-application message; response was voluntary and some questions were only sent to a subset of users, resulting in a large amount of missing data (see online supplemental table 1 for distributions of missing data). Missingness was non-ignorable and was included as a category in our analyses. We categorised age at the start of the first cycle as 18-24, 25-29, 30-34, 35-39, or 40-45 years. Race and ethnic group were reported as Asian, Black, Hispanic, Middle Eastern or North African, Native Hawaiian or Pacific Islander, or white, which we collapsed into a binary variable for modelling. We classified geographical region as UK or Channel Islands, Europe, US or Canada, Australia or New Zealand, and other. Most individuals in the European region were from Sweden (56%) and in the other category, most were from Brazil (62%). We categorised body mass index as underweight (<18.5), normal weight (18.5-24.9), overweight (25.0-29.9), and obese (30.0 or above), combining underweight and normal weight for modelling due to the small sample size of underweight individuals. Additional characteristics included parity (nulliparous v parous), education (at least an undergraduate degree or not), and relationship status (in a relationship or not).
Supplemental material
The Oregon Health and Science University Institutional Review Board approved the protocol (No 00023204), as did Natural Cycles’ research oversight committee, and the Reading Independent Ethics Committee, UK (No 230721). All participants consented to the use of their de-identified data for research, which were used under a data use agreement with Natural Cycles USA.
Statistical analysis
We described sociodemographic characteristics of our sample by vaccination status. We compared all mean changes within individuals in cycle and menses length, by vaccination status, using two sided t tests. We created histograms overlaying vaccination status to compare the distributions of changes in cycle and menses length, and compared the proportion of individuals who had a clinically significant change in cycle length (≥eight days) using Pearson’s χ2 tests. We compared sociodemographic and prevaccination menstrual characteristics of individuals who had a change of eight days or more change to those who did not using two sided t tests and Pearson’s χ2 tests, by vaccination group.
We developed longitudinal multivariable mixed effects models for all cycle and menses length outcomes, and plotted the adjusted lengths (in days) before and after vaccination. Models contained random intercepts and slopes at the individual level, and an interaction term between time (before and after vaccination) and vaccination status to determine the effect of vaccination. The effect was defined as the adjusted difference in the change in cycle and menses length between vaccination groups. All estimates were adjusted for age, body mass index, parity, race or ethnic group, education, relationship status, and global region.
Based on previous findings in the US cohort,15 we performed a subanalysis focused on the number of doses received in a single cycle (one v two). About 5% of the vaccinated sample received their second dose in the same cycle as the first. We stratified vaccinated individuals by the number of doses received in the first dose vaccine cycle and compared all outcomes for each group to the unvaccinated cohort.
We conducted multiple sensitivity analyses to confirm the robustness of our results. Firstly, although the data did not meet the missing at random assumption required for imputation techniques, we performed 500 iterations of imputation followed by weighting with covariate balancing propensity scores and bootstrapped standard errors. We did this analysis to confirm that our results were not biased by missing data or covariate imbalance between vaccination groups.17 We compared the changes in cycle length among vaccinated individuals by the vaccine’s mechanism of action (mRNA, adenovirus vector, or inactivated virus), adjusting for age group to account for age dependent differences in vaccine rollouts. Any individual who reported polycystic ovarian syndrome, thyroid disorder, or endometriosis was excluded (804 individuals). We excluded people who reported use of emergency contraception during at least one study cycle (715 individuals). Additionally, we excluded individuals with any cycle before vaccination whose absolute cycle length was outside of the 24-38 day range (3006 individuals). We also analysed changes during the first vaccine dose cycle, excluding individuals with no data for the second vaccine dose cycle (n=5599) and for the after vaccination cycle (n=6617). Finally, we stratified by global region to examine any potential regional differences.
We had more than 99% power to detect an unadjusted one day difference in cycle length change or 0.5 day difference in menses length change by vaccination status, at a Bonferroni-corrected significance level of 0.007 (99.3% confidence intervals). We accounted for multiple comparisons among the seven primary and secondary outcomes: cycle and menses length for the first and second vaccine dose cycles, cycle length for the cycle after the vaccine, and the proportion of individuals who had a clinically significant change in cycle length (eight days or more) for the first and second vaccine dose cycles. We adjusted all P values to reflect this reduced significance level of 0.007 (see online supplemental file 2 for prespecified analysis plan).
Supplemental material
Patient and public involvement
No members of the public were directly involved in this study, although the research question was developed in response to public reports of menstrual changes after covid-19 vaccination. Natural Cycles informs users of study results through monthly newsletters, via their research library within the application, and through social media channels. The research team uses academic and public dissemination channels to inform the public of the results.
Results
Of 41 504 eligible users, 19 622 individuals who represented 255 086 cycles met the inclusion criteria (figure 2). The final study sample included 14 936 vaccinated and 4686 not vaccinated individuals (table 1). Overall, the cohort was mostly (80.08%) younger than age 35 years (mean age 30 years)from the Europe (33.55%), the UK or Channel Islands (31.71%), and US and Canada (28.59%; see online supplemental table 2 for a complete list of countries in the sample). Two thirds (66.48%) of the vaccinated cohort received the Pfizer-BioNTech covid-19 vaccine, Moderna 17.46%, Oxford-AstraZeneca 9.06%, and Johnson & Johnson 1.89%). Vaccinated individuals were more likely to report a college education or higher (70.57% who were vaccinated, 53.27% who were not vaccinated).
STROBE flow diagram
Characteristics of study participants (n=19 622). Data are number (%)
Cycle length of vaccine dose cycles
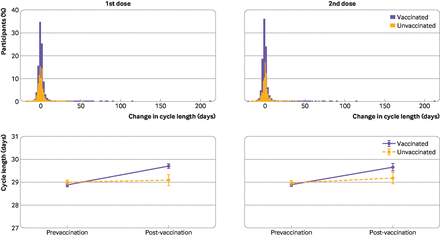
Overall, the vaccinated cohort had a less than one day unadjusted mean increase in the length of their menstrual cycle during the first vaccine dose cycle, compared with their three prevaccination cycles (table 2, 0.81 day increase (99.3% confidence interval 0.68 to 0.93)), whereas the unvaccinated cohort had no significant change in the notional vaccine designated cycle (cycle four) compared with their first three cycles (0.09 day change (99.3% confidence interval −0.08 to 0.27)). Overlaid histograms show a distribution of cycle length change in vaccinated individuals that is roughly equivalent to the unvaccinated individuals with right-hand tails for both groups indicating outliers with increased cycle lengths (figure 3 (top row)). After adjusting for confounders, the difference in the change in cycle length by vaccination status was 0.71 days (figure 3 (bottom row), table 2, 0.47 to 0.96), indicating that vaccination is associated with a less than one day adjusted increase in cycle length.
Mean unadjusted change within individuals in cycle length and menses length, and adjusted difference in the change from three prevaccination cycle average to first or second vaccination cycle and cycle after the second vaccination cycle
(Top row) Overlayed histograms of the change in cycle length (days) between the three prevaccination cycle average and the vaccination cycle for first dose (left) or second dose (right). Histograms for unvaccinated individuals are shown in yellow, vaccinated individuals are shown in purple. (Bottom row) Adjusted marginal means for cycle length (days) for the mean of the three prevaccination cycles and the vaccination cycle for first dose (left) or second dose (right). Estimates are from mixed effects models with random intercepts and random slopes at the individual level, an interaction between vaccination status and timing before and after vaccination, and adjusted for age, body mass index, educational attainment, parity, relationship status, and global region. Error bars represent 99.3% confidence intervals
Individuals who received a second dose (9600 (64.2%) of 14 936 who received a first dose) had an unadjusted mean 0.76 day increase in cycle length during their second vaccine cycle (figure 3 (top row), table 2 (99.3% confidence interval 0.60 to 0.93)), whereas unvaccinated individuals had a smaller but still significant change (0.21 day increase (0.02 to 0.39)). After adjusting for confounders, the difference in the change in cycle length for the second vaccine cycle between unvaccinated and vaccinated was 0.56 days (figure 3 (bottom row), table 2 (0.28 to 0.84)).
The proportion of individuals who had a clinically significant change in cycle length of eight days or more was significantly higher in the vaccinated group during both the first and second vaccine dose cycles (6.2% (929 of 14 936 for the first dose and 597 of 9 600 for the second dose) compared with 5.0% (236 of 4686 for the first dose and 222 of 4423 for the second dose) in the unvaccinated for both cycles; adjusted P=0.019 for the first dose and 0.034 for the second dose). Among the 1342 vaccinated and unvaccinated individuals who had any clinically significant change in cycle length after covid-19 vaccination or notional vaccination date, 523 (38.9%) were only after the first dose, 540 (40.2%) were only after the second dose, and 279 (20.7%) were after both doses. Younger age and longer prevaccination cycle length were associated with clinically significant changes in cycle length in both the vaccinated and unvaccinated groups (online supplemental table 3 for first vaccine dose cycle details).
Cycle length of postvaccine cycle
For vaccinated individuals, the unadjusted cycle length returned to its prevaccination average in the cycle after the second dose, designated as the postvaccine cycle (table 2, 0.09 (99.3% confidence interval −0.03 to 0.20)). The unvaccinated cohort had a small but significant increase similar to the second dose vaccine cycle (0.20 (0.01 to 0.39)). After adjusting for confounders, no significant difference was reported in the change in cycle length between the vaccination groups (–0.11 (−0.33 to 0.10)), indicating resolution of cycle changes associated with vaccination. See online supplemental figure 1 for overlaid histograms and plots of marginal means.
Subpopulation: two doses of vaccine received in one cycle
The increase in cycle length for both first and second vaccine dose cycles appears to be largely driven by individuals who received both vaccine doses within a single cycle (cycle four). This subgroup (n=743) had an almost four day unadjusted mean cycle length increase (table 3, 3.91 days (99.3% confidence interval 2.53 to 5.28)) and 13.5% (100 of 743) had an increase in cycle length of eight days or more versus 5.0% (236 of 4686) unvaccinated (adjusted P<0.001). In adjusted models, individuals who received both vaccine doses in one cycle had a 3.70 day increase in cycle length compared with unvaccinated individuals ((99.3% confidence interval 2.98 to 4.42); table 3). When these individuals were removed from the analysis, both unadjusted and adjusted increases in cycle length for first and second doses in separate cycles were smaller (table 3), and no significant differences were noted in the proportion of individuals with a change in cycle length of eight days or more compared with unvaccinated individuals (5.8% (829 of 14 193) for first dose vaccinated v 5.0% (236 of 4686) unvaccinated, P=0.27; 5.6% (497 of 8857) for second dose vaccinated v 5.0% (222 of 4423) unvaccinated, P=1.00)).
Mean unadjusted within-individual change in cycle length, and adjusted difference in the change from three prevaccination cycle average to first or second vaccination cycle and cycle following the second vaccination cycle, stratified by the number of doses received in a single cycle
In the vaccine cycle after the second dose, the adjusted cycle length change for individuals who received one dose per cycle did not differ from the unvaccinated cohort (table 3); the adjusted difference for those who received both doses in one cycle was attenuated, but still increased compared with the unvaccinated group (0.65 days (99.3% confidence interval 0.13 to 1.18)). These differences do not appear to be driven by individuals with naturally longer cycle lengths who might seem more likely to receive both doses in a single cycle: among the 743 individuals who received two doses in a single cycle, only 48 (6.5%) received their second dose outside of our defined normal cycle length range of 24-38 days.
Menses length
We found no changes in unadjusted menses length for either first or second vaccine dose cycles among vaccinated individuals (table 2, online supplemental figure 2). Adjusted differences in menses length changes between vaccination statuses were not significant for the first dose vaccine cycle (0.07 days (99.3% confidence interval 0.00 to 0.13)). In the second dose vaccine cycle, we found a small but significant adjusted difference (0.13 days (0.06 to 0.20)), which was driven by a decrease in menses length among unvaccinated individuals. Stratification by people who received both doses in one cycle did not change results for menses length.
Sensitivity analyses
Sensitivity analyses implementing imputation and sample weighting, excluding individuals with gynaecological disorders, emergency contraception use, more variable prevaccination cycle lengths, or without data for the second dose or postvaccine cycles, and stratifying by global region did not alter our results in a clinically meaningful way (online supplemental table 4 for imputation and weighting results). Changes in cycle length, adjusted for age group, did not differ substantially by a vaccine’s mechanism of action (mRNA, adenovirus vector, or inactivated virus; online supplemental figure 3).
Discussion
Principal findings
We report more than a quarter of a million menstrual cycles, prospectively recorded, by almost 20 000 individuals. Compared with the unvaccinated group, vaccinated individuals had an adjusted increase in menstrual cycle length of less than one day with both first and second vaccine doses. Individuals who received two doses of a covid-19 vaccine in a single cycle had an adjusted increase in cycle length of 3.70 days compared with the unvaccinated. Additionally, a significant increase was noted in the proportion of respondents who had an increase in cycle length of more than eight days (13.5%, compared with 5.0% in the unvaccinated cohort). Cycle length changes did not remain in the cycle after vaccination, except in the group that received two vaccine doses in one cycle, where cycle length changes were attenuated but still increased compared with the unvaccinated group. Cycle length changes due to covid-19 vaccination appear similar across the different vaccine types. We found no differences in menses length in any group of vaccinated individuals, compared with the unvaccinated cohort.
Comparison with other studies
Our findings are consistent with those from our previous publication of a US only cohort and provide further evidence of small cycle length changes associated with covid-19 vaccination.15 This study represents a larger, more geographically diverse population receiving a broader range of vaccine types and brands as well as differing vaccine timing schemes than our previous publication. Although local factors (eg, vaccine rollout and dosing guidelines) could have resulted in divergent findings from the US only cohort, the findings appear consistent.3 15 Of note, these analyses include data represented in our published US cohort but as eligibility criteria differed between the two analyses we were able to add an additional 1000 US based individuals. Additionally, we were able to assess resolution of menstrual changes in the cycle postvaccine and perform sensitivity analyses of vaccine type. We found that cycle length changes associated with mRNA vaccines do not appear to differ from those with other vaccine mechanisms, which can reassure people with concerns about this newer vaccine technology.
Of note, we did observe some cycle changes between the notional prevaccine and postvaccine cycles in the unvaccinated cohort. These findings reflect the fact that menstrual cycles vary, even in the absence of vaccination, and underline the need for the inclusion of an unvaccinated comparison group and baseline measures of menstrual outcomes to determine the extent to which any change observed can be attributed to vaccination. Our research was not designed to determine why these changes might happen; these changes are probably due to temporary vaccine-related activation of immune response but more research is needed.
Strengths and limitations of this study
Strengths of our study include robust study design and analytical methodology, the inclusion of an unvaccinated comparison group, and prospectively tracked menstrual cycle data. These items altogether mean that our outcomes are not affected by recall bias, either due to cross-sectional documentation of vaccine dosage and outcomes or the natural variation in menstrual outcomes. Our sample size is sufficiently large to identify small differences, which is critically important to public stakeholders. However, this study has some important limitations. Our data include individuals not using hormonal contraception with regular menstrual cycles prevaccine between 18 and 45 years old. This decision was purposeful in order to assess the effects related to the vaccine. We recognise that many individuals who menstruate might not be represented in these analyses and people with greater baseline menstrual disturbances, including those at age and body mass index extremes, gynaecological disorders, or hormonal contraceptive users, might have a different experience. Our dataset is limited in its number of cycles postvaccine to assess resolution of cycle length changes among individuals receiving two doses per cycle. We are also unable to account for the potential effect of covid-19 infections that both unvaccinated and vaccinated participants might have contracted during the study on menstrual cycle outcomes. Our deidentified data are self-reported, however, this method is the gold standard for menstrual cycle data. Our study design does not permit us to verify vaccine status or dates but this information is readily available for most individuals. Finally, although we implemented a rigorous study design and analytical method, the possibility of residual confounding and bias exists.
Conclusions
Our findings from this large international cohort of individuals continue to be reassuring and can be used to counsel individuals about what to expect with a covid-19 vaccination and how to make an informed decision about vaccination versus continuing to be at risk for covid-19 disease and its related morbidity and mortality. Although we do find menstrual changes after covid-19 vaccination, these changes are small compared with normal variation and resolve in the cycle after vaccination, except in people who received both doses in one menstrual cycle. Future work should assess other aspects of changes to menstrual cycles, such as unexpected vaginal bleeding, menstrual flow and pain, and define the mechanism by which the postvaccination menstrual changes described here occur.
Data availability statement
No data are available. Data were accessed under a data use agreement with Natural Cycles USA Corp, New York and are not available to third parties.
Ethics approval
Not applicable.
Acknowledgments
We thank the individuals who reported their menstrual experiences around the covid-19 vaccine: their voices informed our work. Additionally, we acknowledge the contribution to science that would not have been possible without the permission of the users of Natural Cycles to share their de-identified data.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors AE, BGD, and ERB report substantial contributions to the design, dataset preparation and readiness, analysis, interpretation of the results, drafting the work, and approval of the version to be published. EB, JTP, and AVL report substantial contributions to the design, acquisition and interpretation of the data, revising it critically for important intellectual content, and final approval of the version to be published. KAM, VM, and STC report substantial contributions to the design or the work, interpretation of results, revising the work critically for important intellectual content, and final approval of the version to be published. All authors are accountable for all aspects of the work; AE serves as the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Transparency: The lead author (the guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Funding Research reported in this publication was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the NIH Office of Research on Women's Health. NIH NICHD089957 Supplement. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests AE reports honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, World Health Organization, and Gynuity for committee activities and honorariums for peer review from the Karolinska Institute. AE receives royalties from UptoDate. Oregon Health and Science University (OHSU) receives research funding from OHSU Foundation, Merck, HRA Pharma, and the National Institutes of Health for which Alison Edelman is the principal investigator. BGD reports honorariums and travel reimbursement from the American College of Obstetricians and Society of Family Planning for board, committee, and mentorship activities. OHSU receives research funding from Merck/Organon and Office of Population Affairs/Department of Health and Human Services for which BGD is the principal investigator. OHSU receives research funding from OHSU foundation, the Bill & Melinda Gates Foundation, American Board of Obstetrics and Gynecology, American Society for Reproductive Medicine, and the National Institutes of Health for which Leo Han is the principal investigator. EB, AVL, and JTP are employees of Natural Cycles. Natural Cycles received cost reimbursement from grant funds for data processing and secure transfer. KAM reports honorariums and travel reimbursement from the American Board of Obstetrics and Gynecology and travel reimbursement from American College of Obstetricians and Gynecologists. Women and Infants Hospital received funding from Myovant for consulting work done by KAM on outcomes measures for heavy menstrual bleeding. VM reports research funding from Borne, payment for acting as an external examiner for the Universities of Cambridge, Leeds and Swansea, and royalties received for contribution to Immunology 9th edition (Elsevier). STC is the editor-in-chief of BMJ Sexual and Reproductive Health and reports an honorarium from Gedeon Richter Nordics for a lecture on contraception. ERB declares no competing interests.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.