Abstract
No specific antiviral drugs have been approved for the treatment of COVID-19. This study aimed to evaluate the efficacy of favipiravir in treatment of COVID-19. This was a multicenter randomized controlled study including 96 patients with COVID- 19 who were randomly assigned into a chloroquine (CQ) group and a favipiravir group. None of the patients in the favipiravir group needed mechanical ventilation (p = 0.129). One patient (2.3%) in the favipiravir group and two patients (4.2%) in the CQ group died (p = 1.00). Favipiravir is a promising drug for COVID-19 that decreases the hospital stay and the need for mechanical ventilation.
ClinicalTrials.gov Identifier NCT04351295.
Introduction
COVID-19 has led to a major worldwide health and economic crisis, with more than 27 million people having contracted the disease and more than 800,000 deaths [1, 2]. No specific antiviral drugs have been approved for the treatment of COVID-19 [3].
The food and drug administration (FDA) granted emergency approval to allow hospitals to use chloroquine and hydroxychloroquine for treatment of COVID-19, and these drugs have been used as standard of care in some countries [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. However, many questions remain about the efficacy of chloroquine in treatment of COVID-19 [6,7,8,9,10,11,12,13,14,15,16]
Other antiviral drugs have been suggested to be repurposed for the treatment of COVID-19, such as interferon-ɑ, lopinavir/ritonavir, ribavirin, and remdesivir [5, 6]. Some clinical trials concentrating on viral RNA-dependent RNA polymerase (RdRp) inhibitors have been registered and started [6,7,8,9,10,11]. Favipiravir, a purine analogue and a potent RdRp inhibitor that has been approved for use in influenza treatment, is also being considered for treatment of COVID-19 [7,8,9,10,11].
Favipiravir acts as a purine analogue and is incorporated in place of guanine or adenine [7,8,9,10] and thereby inhibits viral replication. It has been used for treatment of some life-threatening infections such as Ebola, Lassa fever, and rabies, and its therapeutic usefulness has been established in these diseases [8,9,10,11].
Data about the efficacy of favipiravir in the treatment of COVID-19 are very scarce. Therefore, the aim of the study was to evaluate the efficacy of favipiravir in treatment COVID-19.
Methods
This was a multi-center, randomized, interventional phase2/phase3 study that included 96 patients with confirmed SARS-CoV-2 infection. This study was performed at the Ain-Shams University and Tanta University hospitals in the period from April to August 2020. Ethical approval was obtained from the Tanta University Faculty of Medicine Ethics Committee, and the approval number was 34035/20. The study was registered on clinicaltrials.gov under the registration number NCT04351295.
Patients who met the criteria to be included in the study were enrolled. The criteria for inclusion included being an adult 18 to 80 years of age with confirmed SARS-CoV-2 infection with mild or moderate symptoms and having been admitted to the hospital three days after the onset of symptoms. All of the patients agreed to participate in the study and signed an informed consent statement.
Patients who had allergy or contraindication to the drug, pregnant and lactating mothers, and patients with cardiac problems, liver or renal failure, or other organ failure were excluded from the study.
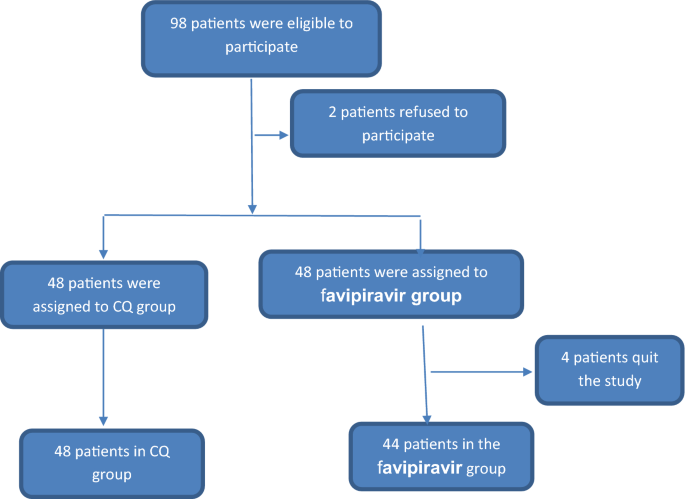
Ninety-eight patients were eligible to participate in the study. After exclusion of patients who refused to participate, 96 patients were randomly assigned into two groups. The chloroquine (CQ) group included 48 patients who received chloroquine 600 mg tablets twice daily added to the standard-of-care therapy for 10 days. The favipiravir group included 48 patients who received 1600 mg of favipiravir twice a day on the first day and 600 mg twice a day from the second to tenth day, added to the standard-of-care therapy for 10 days. Four patients in this group quit after the beginning of the study, and the final number in this group was 44 patients. The four patients who left the study preferred to complete their treatment and be transferred to military hospitals, after which we lost contact with them (Fig. 1).
All participants in the study were interviewed and their demographic and basal data were recorded, including age, sex, weight, height, and body mass index (BMI). All of the patients were subjected to a thorough clinical examination, and blood samples were taken for biochemical analysis, including a complete blood count (CBC), liver function tests, renal function tests, chest X-ray, chest CT scan, and ECG. The principal outcomes of the study were the mortality rate and the need for mechanical ventilation.
Sample size calculation was done using G*power software. A study done by Cai et al. [17] showed that antiviral therapy with favipiravir was able to reduce the time of viral clearance from 11 days to 4 days (about 63%). Based on that study, with a sample power of 80%, an α error of 0.05, and an allocation ratio of 1, the sample size was at least 43 patients in each group [18].
Statistical analysis: The normality of the different variables was tested by Shapero Wilks test. Continuous variables were expressed as the mean, SD and median, while the categorical variables were expressed as numbers and percentages. Student’s t-test was used for normally distributed quantitative variables, while the Mann-Whitney test was used for non-normally distributed ones. The chi-square test (χ2) was used for categorical variables and whenever any of the expected cells were less than five, Fischer’s exact test was used. Univariate binary logistic regression was used to ascertain the effect of possible risk factors on the overall mortality of the patients. A two-sided P-value less than 0.05 was considered statistically significant. The analysis was done with SPSS Statistical Package version 23 (SPSS Inc. Released 2015. IBM SPSS Statistics for Windows, version 23.0, Armnok, NY, IBM Corp.).
Results
The two groups were matched for gender and age (p = 0.525 and 0.717, respectively). There was no significant difference regarding laboratory parameters, including hemoglobin, WBCs, platelets, CRP, ferritin, D dimer, ALT, AST, or creatinine. There was also no significance difference between the two groups regarding comorbidities (Table 1).
Although not statistically significant (p = 0.06), the favipiravir group had a lower mean duration of hospital stay (13.29 ± 5.86 days) than the CQ group (15.89 ± 4.75 days). None of the patients in the favipiravir group needed mechanical ventilation or had an oxygen saturation lower than 90%, but in comparison to the CQ group, these differences were not significant (p = 0.118 and 0.129, respectively). Four patients in the CQ group needed mechanical ventilation and received methylprednisolone after their condition worsened. Two patients (4.2%) in the CQ group and one (2.3%) in the favipiravir group died (p = 1.00, Table 2). No significant differences were observed between the groups regarding side effects (Table 2).
Univariate logistic regression of possible risk factors for overall mortality revealed that the patient’s age and CRP level were the only factors significantly associated with mortality (p = 0.045 and 0.019, respectively). Favipiravir treatment was not significantly associated with COVID-19 mortality in this study (p = 0.615).
Discussion
The current epidemic of COVID-19 has reached pandemic proportions, and intense public health efforts are under way to contain the epidemic worldwide. However, as conclusive therapies for proven COVID-19 continue to be a challenge, there is a considerable interest in repurposing existing antiviral agents [19]. Favipiravir (FPV) is a novel RdRp inhibitor that has been demonstrated to be efficient in treating influenza and Ebola virus infections [20, 21].
Favipiravir is a prodrug that is ribosylated and phosphorylated intracellularly to form the active metabolite favipiravir ibofuranosyl‐5′‐triphosphate (T‐705‐RTP). T‐705‐RTP competes with purine nucleosides and interferes with viral replication by getting incorporated into the viral RNA and thereby inhibiting the RdRp of RNA viruses [24,25,26,27,28].
In this randomized multicenter study, the patients who received FPV had a lower mean duration of hospitalization than the CQ group. None of the patients in the FPV group needed mechanical ventilation, in contrast to the CQ group, but these results were not statistically significant. This is a potentially important observation, as decreasing the need for mechanical ventilation among COVID-19 patients is crucial, especially in developing countries and regions of the world with limited resources.
Two patients (4.2%) in the CQ group and one (2.3%) in the FPV group died. This finding suggests that improvement of the patient's condition may depend on inhibition of SARS-CoV-2 and that FPV controls the disease progression of COVID-19 by inhibiting SARS-CoV-2 polymerase activity [9].
To our knowledge, this is the first randomized study to evaluate the efficacy of favipiravir for treatment of COVID-19.
The positive results of this study are supported by three previous case reports. The first was by Noda et al., who reported the cases of two elderly COVID-19-positive patients, one of whom had hypoxemia, who received favipiravir with a seemingly beneficial effect [22]. The second case report described a case of COVID-19 pneumonia that did not worsen and was relieved by early administration of favipiravir and ciclesonide [23]. The third report described administration of a combination of FPV with short-course systemic corticosteroid treatment to a patient who was critically ill with COVID-19 pneumonia and COPD who subsequently showed improvement [28]. Although these data support our finding, they are case reports that need to be verified in large randomized controlled studies.
A non-randomized interventional study involving 80 patients with non-severe COVID-19 compared favipiravir with lopinavir/ritonavir and showed increased viral clearance in the favipiravir group on day 7, supporting the possible applicability of favipiravir in treatment of COVID-19 [17].
A positive effect of favipiravir was also suggested in a case series by Doi et al., who used a combination of favipiravir and nafamostat mesylate, which showed promising results in critically ill COVID-19 patients [27].
The dose of FVP to be given to critically ill patients is controversial, especially since the publication of recent data showing lower serum levels of the drug in these patients than in less severely ill patients [26].
The main limitation of this study is that it was based on clinical outcomes, the need for ICU admission, and mortality and that the viremic response was not investigated. This was due to the limited resources available. Also, the study included only COVID-19 patients who were mildly or moderately ill and therefore had a better prognosis than severely or critically ill patients.
In conclusion, favipiravir is a promising drug for treatment of COVID-19 that might decrease the hospital stay and the need for mechanical ventilation.
Change history
03 August 2021
Editor's Note:The Editor-in-Chief is currently investigating this article as concerns have been raised about the reporting of this clinical trial. Further editorial action will be taken as appropriate once the investigation into the concerns is complete and all parties have been given an opportunity to respond in full.
22 November 2021
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00705-021-05307-4
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 382(8):727–733
Yin Y, Wunderink RG (2018) MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 23(2):130–137
Cascella M, Rajnik M, Cuomo A et al (2012) Features, evaluation and treatment coronavirus (COVID-19). StatPearls Publishing, Treasure Island
Yazdany J, Kim AHJ (2020) Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med. 172:754–755
Hernandez AV, Roman YM, Pasupuleti V et al (2020) Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 173(4):287–296
Du YX, Chen XP (2020) Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther 108(2):242–247
Arab-Zozani M, Hassanipour S, Ghoddoosi-Nejad D (2020) Favipiravir for treating patients with novel coronavirus (COVID-19): protocol for a systematic review and meta-analysis of randomised clinical trials. BMJ Open. 10(7):e039730
Goldhill DH, Te Velthuis AJ, Fletcher RA et al (2018) The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci 115(45):11613–11618
Zhu W, Chen CZ, Gorshkov K et al (2020) RNA-dependent RNA polymerase as a target for COVID-19 Drug discovery. SLAS DISCOV Adv Sci Drug Discov. https://doi.org/10.1177/2472555220942123
Elfiky AA (2020) SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1761882
Mohamed AA, Mohamed N, Mohamoud S et al (2020) SARS-CoV-2: the path of prevention and control. Infect Disord Drug Targets. https://doi.org/10.2174/1871526520666200520112848 (Online ahead of print)
Sarin SK, Choudhury A, Lau GK et al (2020) Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int 14(5):690–700. https://doi.org/10.1007/s12072-020-10072-8
Abd-Elsalam S, Elkadeem M, Glal KA (2020) Chloroquine as chemoprophylaxis for COVID-19: Will this work? Infect Disord Drug Targets. https://doi.org/10.2174/1871526520666200726224802 (Online ahead of print)
Abd-Elsalam S, Esmail ES, Khalaf M et al (2020) Tanta protocol for management of COVID-19. Perspectives from a developing country. Endocr Metab Immune Disord Drug Targets. https://doi.org/10.2174/1871530320999201117142305 (Online ahead of print)
Xie M, Chen Q (2020) Insight into 2019 novel coronavirus—an updated intrim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. https://doi.org/10.1016/j.ijid.2020.03.071 ([Epub ahead of print])
Marjot T, Moon AM, Cook JA et al (2020) Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. https://doi.org/10.1016/j.jhep.2020.09.024 (Online ahead of print. PMID: 33035628)
Cai Q, Yang M, Liu D et al (2020) Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing). https://doi.org/10.1016/j.eng.2020.03.007 ([Epub ahead of print])
Faul F, Erdfelder E, Lang A-G et al (2007) A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
World Health Organization. Coronavirus disease (COVID-19) situation report—139. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200607-covid-19-sitrep-139.pdf?sfvrsn=79dc6d08_2. Accessed 8 June 2020.
Oestereich L, Lüdtke A, Wurr S et al (2014) Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 105:17–21
Madelain V, Oestereich L, Graw F et al (2015) Ebola virus dynamics in mice treated with favipiravir. Antiviral Res 123:70–77
Noda A, Shirai T, Nakajima H et al (2020) Case report: two cases of COVID-19 pneumonia including use of favipiravir. The Japanese Association for Infectious Diseases. http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_en_200408_2.pdf
Yokoyama K, Oguri T, Kato A et al (2020) Case report a case of COVID-19 pneumonia that did not worsen and was relieved by early administration of favipiravir and ciclesonide. http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_en_200406.pdf.
Abena PM et al (2020) Chloroquine and hydroxychloroquine for the prevention or treatment of COVID-19 in Africa: caution for inappropriate off-label use in healthcare settings. Am J Trop Med Hyg 102:1184–1188
Furuta Y et al (2005) Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 49:981–986
Irie K, Nakagawa A, Fujita H et al (2020) Pharmacokinetics of favipiravir in critically Ill patients with COVID-19. Clin Transl Sci. 13(5):880–885
Doi K, Ikeda M, Hayase N et al (2020) Nafamostat mesylate treatment incombination with favipiravir for patients critically ill with Covid-19: a case series. Crit Care 24:392
Inoue H, Jinno M, Ohta S et al (2020) Combination treatment of short-course systemic corticosteroid and favipiravir in a successfully treated case of critically ill COVID-19 pneumonia with COPD. Respir Med Case Rep 31:101200
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Handling Editor: Zhongjie Shi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://dx.doi.org/10.1007/s00705-021-05307-4
About this article
Cite this article
Dabbous, H.M., Abd-Elsalam, S., El-Sayed, M.H. et al. RETRACTED ARTICLE: Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Arch Virol 166, 949–954 (2021). https://doi.org/10.1007/s00705-021-04956-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-021-04956-9