Abstract
Imprinted genes are expressed differently depending on whether they are carried by a chromosome of maternal or paternal origin. Correct imprinting is established by germline-specific modifications; failure of this process underlies several inherited human syndromes1,2,3,4,5. All these imprinting control defects are cis-acting, disrupting establishment or maintenance of allele-specific epigenetic modifications across one contiguous segment of the genome. In contrast, we report here an inherited global imprinting defect. This recessive maternal-effect mutation disrupts the specification of imprints at multiple, non-contiguous loci, with the result that genes normally carrying a maternal methylation imprint assume a paternal epigenetic pattern on the maternal allele. The resulting conception is phenotypically indistinguishable from an androgenetic complete hydatidiform mole6, in which abnormal extra-embryonic tissue proliferates while development of the embryo is absent or nearly so. This disorder offers a genetic route to the identification of trans-acting oocyte factors that mediate maternal imprint establishment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sutcliffe, J. S. et al. Deletions of a differentially methylated CpG island at the SNRPN gene define a putative imprinting control region. Nature Genet. 8, 52–58 (1994).
Buiting, K. et al. Inherited microdeletions in the Angelman and Prader-Willi syndromes define an imprinting centre on human chromosome 15. Nature Genet. 9, 395–400 (1995).
Reik, W. et al. Imprinting mutations in the Beckwith-Wiedemann syndrome suggested by altered imprinting pattern in the IGF2-H19 domain. Hum. Mol. Genet. 4, 2379–2385 (1995).
Gardner, R. J. et al. An imprinted locus associated with transient neonatal diabetes mellitus. Hum. Mol. Genet. 9, 589–596 (2000).
Liu, J. et al. A GNAS1 imprinting defect in pseudohypoparathyroidism type IB. J. Clin. Invest. 106, 1167–1174 (2000).
Kajii, T. & Ohama, K. Androgenetic origin of hydatidiform mole. Nature 268, 633–634 (1977).
Moglabey, Y. B. et al. Genetic mapping of a maternal locus responsible for familial hydatidiform moles. Hum. Mol. Genet. 8, 667–671 (1999).
Helwani, M. N. et al. A familial case of recurrent hydatidiform molar pregnancies with biparental genomic contribution. Hum. Genet. 105, 112–115 (1999).
Fisher, R. A., Khatoon, R., Paradinas, F. J., Roberts, A. P. & Newlands, E. S. Repetitive complete hydatidiform mole can be biparental in origin and either male or female. Hum. Reprod. 15, 594–598 (2000).
Clark, S. J., Harrison, J., Paul, C. L. & Frommer, M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 22, 2990–2997 (1994).
Kerjean, A. et al. Establishment of the paternal methylation imprint of the human H19 and MEST/PEG1 genes during spermatogenesis. Hum. Mol. Genet. 9, 2183–2187 (2000).
Lee, M. P. et al. Loss of imprinting of a paternally expressed transcript, with antisense orientation to KVLQT1, occurs frequently in Beckwith-Wiedemann syndrome and is independent of insulin-like growth factor II imprinting. Proc. Natl Acad. Sci. USA 96, 5203–5208 (1999).
Ohta, T. et al. Imprinting-mutation mechanisms in Prader-Willi syndrome. Am. J. Hum. Genet. 64, 397–413 (1999).
Engemann, S. et al. Sequence and functional comparison in the Beckwith-Wiedemann region: implications for a novel imprinting centre and extended imprinting. Hum. Mol. Genet. 9, 2691–2706 (2000).
Shemer, R., Birger, Y., Riggs, A. D. & Razin, A. Structure of the imprinted mouse Snrpn gene and establishment of its parental-specific methylation pattern. Proc. Natl Acad. Sci. USA 94, 10267–10272 (1997).
El-Maarri, O. et al. Maternal methylation imprints on human chromosome 15 are established during or after fertilization. Nature Genet. 27, 341–344 (2001).
Kaneko-Ishino, T. et al. Peg1/Mest imprinted gene on chromosome 6 identified by cDNA subtraction hybridization. Nature Genet. 11, 52–59 (1995).
Murphy, S. K., Wylie, A. A. & Jirtle, R. L. Imprinting of PEG3, the human homologue of a mouse gene involved in nurturing behavior. Genomics 71, 110–117 (2001).
Hayward, B. E. et al. The human GNAS1 gene is imprinted and encodes distinct paternally and biallelically expressed G proteins. Proc. Natl Acad. Sci. USA 95, 10038–10043 (1998).
Hayward, B. E., Moran, V., Strain, L. & Bonthron, D. T. Bidirectional imprinting of a single gene: GNAS1 encodes maternally, paternally, and biallelically derived proteins. Proc. Natl Acad. Sci. USA 95, 15475–15480 (1998).
Hayward, B. E. & Bonthron, D. T. An imprinted antisense transcript at the human GNAS1 locus. Hum. Mol. Genet. 9, 835–841 (2000).
Liu, J., Yu, S., Litman, D., Chen, W. & Weinstein, L. S. Identification of a methylation imprint mark within the mouse Gnas locus. Mol. Cell Biol. 20, 5808–5817 (2000).
Smilinich, N. J. et al. A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith-Wiedemann syndrome. Proc. Natl Acad. Sci. USA 96, 8064–8069 (1999).
Kamiya, M. et al. The cell cycle control gene ZAC/PLAGL1 is imprinted—a strong candidate gene for transient neonatal diabetes. Hum. Mol. Genet. 9, 453–460 (2000).
Reik, W. & Walter, J. Evolution of imprinting mechanisms: the battle of the sexes begins in the zygote. Nature Genet. 27, 255–256 (2001).
Bourc’his, D., Xu, G.-L., Lin, C.-S., Bollman, B. & Bestor, T. H. Dnmt3L and the establishment of maternal genomic imprints. Science 294, 2536–2539 (2001); advance online publication, 29 November 2001 (DOI 10,1126/Science.1065848).
Strain, L., Warner, J. P., Johnston, T. & Bonthron, D. T. A human parthenogenetic chimaera. Nature Genet. 11, 164–169 (1995).
Acknowledgements
We thank R. Fisher for supplying androgenetic CHM DNAs, and G. Taylor for Prader–Willi and chorionic villus sample DNA samples. This work was supported by the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests
Supplementary information
Detailed methods
Methylation analysis of differentially methylated regions (DMRs) of imprinted genes
DNA (1 µg / 50 µl) was digested overnight with 25 units of Pvu II (or Bam HI in the case of SNRPN , where a Pvu II site lies between the primers). Ethanol-precipitated products were resuspended in 50 µl TE buffer (pH 8.0). Following denaturation (with 5.5 µl 3M NaOH at 37°C for 15 minutes), 30 µl 10 mM hydroquinone, and 520 µl (3.6M) sodium bisulphite pH 5.0 were added. The reactions were incubated at 55°C for 16 hours. DNA was purified with GenecleanTM (Bio101), and eluted into 20 µl water. A further denaturation was performed by adding 2.2 µl 3M NaOH and incubating at 37°C for 15 minutes. The denatured modified DNA was ethanol-precipitated and resuspended in 15 µl TE; 2 µl was used per 50 µl PCR reaction. PCR primer sequences and cycling conditions were as follows:
H19: dTGTATAGTATATGGGTATTTTTGGAGGTTT and dTCCTATAAATATCCTATTCCCAAATAACC (Kerjean et al., ref. 11 in main text). 35 cycles of 94, 52, 72ºC; 45, 45, 90 s. Sequencing primer dTTTTGGTTTTATTGTTTGGATGGTA. The region sequenced was nt 6115-6326 of AF087017.
KCNQ1OT1 (LIT1): dTGTTGAGGAGTTTYGGGGAGGATTA and dCACCTCACACCCAACCAATACCTCAT (40 cycles of 94, 50, 72ºC; 45, 45, 60 s). Sequenced with dTTGGTAGGATTTTGTTGAGGAGTTTT or dAAACCAAACTCTTTCAACCAATAAC.
SNRPN: Nested PCR1. First round: dGGTTTTTTTTTATTGTAATAGTGTTGTGGGG and dCTCCAAAACAAAAAACTTTAAAACCCAAATTC. Second round: dCAATACTCCAAATCCTAAAAACTTAAAATATC and dGGTTTTAGGGGTTTAGTAGTTTTTTTTTTTTTGG (also used as a sequencing primer). 35 cycles of 94, 51, 72ºC; 60, 60, 60 s. 2.5 µl were then diluted into a fresh 50 ml reaction and reamplified for a further 25 cycles.
PEG1: dtygttgttggttagttttgtayggtt and dAAAAATAACACCCCCTCCTCAAAT (Kerjean et al., ref. 11 in main text). 40 cycles of 94, 61, 72ºC; 45, 45, 60s. Sequencing primer dAAAATTTCRACCCAAAAACAACCCC.
ZIM2/PEG3: dAAAAGGTATTAATTATTTATAGTTTGGT and dAAAAATATCCACCCTAAACTAATAA (also used as a sequencing primer). 6 cycles of 94ºC 30s, -1ºC/s ramp to 60ºC, 60ºC 20s (-1ºC/cycle), 72ºC 60s; 6 cycles of 94ºC 30s, -1ºC/s ramp to 55ºC, 55ºC 20s (-1ºC/cycle), 72ºC 60s; 32 cycles of 94ºC 30s, 1ºC/s ramp to 50ºC, 50ºC 20s, 72ºC 60s.
GNAS1. The positions of each amplicon were: exon 1A, nt 29821-30039 of AL121917; NESP55, 106677-107019 of AL132655; XL as, 120557-120954 of AL132655; antisense promoter, 117154-117607 of AL132655.
GNAS1- 1A: Nested PCR. First round (35 cycles of 94, 55, 72ºC; 45, 45, 90 s): dTTTTGTTTTTTTTTYGTTTGTTTAT and dACAACTTCRACAACCACCTCRACAAC. Second round (25 cycles using 2 µl of first round products): dTAAACTTCATAACCATCTTCAACATAA and dTTAATTTTTAGGTAGTTAGTTTAGTAGTT (also used as a sequencing primer).
GNAS1- NESP55: dTTTTTGTAGAGTTAGAGGGTAGGT (also used as a sequencing primer), and dAAATAAAACAACTCAAAATCTACC (40 cycles of 94, 50, 72ºC; 45, 45, 60 s).
GNAS1- antisense: dTATTTGTGTAGGTTTAGTATTTTTGG and dCATCCTCTAAATAACCCAACTAAATC (40 cycles of 94, 54, 72ºC; 45, 45, 60 s). Sequencing primer: dAAACAAAAATCATACCAATCAAAAC.
GNAS1- XL as: dGTTGGTTTTAGAGGAGGTTATAGTT and dCCTCCTCAACTAAAAATCTCTCTAC (40 cycles of 94, 54, 72ºC; 45, 45, 60 s). Sequencing primer: dGTTATTTGAGTTTGATGGAGAAGG.
KHK: dTTTAGTAGTTTTGTTTTGGATGATT and dACAACTAACAAAACACAAACAATTA (8 cycles of 94ºC 30s, 0.5ºC/s ramp to 59ºC, 59ºC [-1ºC/cycle] 30s, 72ºC 60s; 32 cycles of 94ºC 30s, 0.5ºC/s ramp to 50ºC, 50ºC 30s, 72ºC 60s).
PCR products were treated with shrimp alkaline phosphatase and exonuclease I, and cycle-sequenced with a Thermosequenase 33P-labelled terminator kit (AmershamPharmacia). dITP was needed to reduce the effect of GC compressions, which otherwise cause differential migration of the allelic sequences on the sequencing gel. Cycling conditions were 50 cycles of 95ºC for 30 s, 50ºC for 30 s, and 60ºC for 7 minutes.

Figure 1. Pedigree of BiCHM family.
(GIF 13.9 KB)
Filled diamonds indicate molar pregnancies. The index case IV:4 is arrowed. Known consanguineous matings are indicated by double bars. Filled circles indicate affected women (presumed homozygous by descent for the causative mutation), who are, however, themselves phenotypically normal except during pregnancy. DNA from affected individuals IV:4 and III:14 was used for genetic analysis.

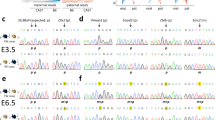
Figure 2. Clonal analysis of bisulphite-PCR products from BiCHM
(GIF 30.17 KB)
Methylation analysis was mostly performed as described above by direct sequencing of bisulphite-PCR products, since this avoids problems of cloning bias at some loci. However, some loci displaying a mixed methylation pattern on direct sequencing were analysed by cloning to assess the allele-specificity of this methylation. One clone per horizontal line; black symbol = methylated residue; white symbol, unmethylated residue. a, H19 . Labelling of CpGs is as in Kerjean et al., ref. 11 in main text. The distribution of methylated and unmethylated CpGs is indistinguishable from that in normal somatic tissues. It is consistent with unimpaired establishment and maintenance of a paternal imprint at H19 in the BiCHM. b, GNAS1-XL a s. This, as discussed in the main text, was the only maternally methylated DMR not to show clear evidence of a defect in the BiCHM on direct sequencing. This retention of differential methylation has also been noted in some PHP-Ib patients with cis- acting imprinting mutations at this locus. However, the cloned BiCHM GNAS1-XL products show a strikingly different appearance from that of H19. This suggests that the appearance on direct sequencing does not reflect normal imprinting at GNAS1-XL , but a rather disordered scattering of methylated residues across both alleles

Figure 3. Clonal analysis of bisulphite-PCR products from AgCHM.
(GIF 39.91 KB)
At several loci, the BiCHM methylation pattern is indistinguishable from that of AgCHM (H19 representing an important predicted exception). However, at four DMRs (PEG1, KCNQ1OT1, SNRPN and GNAS1- 1A), the BiCHM epigenotype actually appears even more purely “paternal” than that of the AgCHM. The reason for this is unclear, though it implies that the presence of an extra paternal allele (rather than an abnormal maternal one) increases the susceptibility of the CHM to acquiring abnormal postzygotic methylation changes. The failure of AgCHM to conform perfectly to the expected paternal methylation pattern has been reported previously2. There is also evidence that early uniparental mouse embryos may “adjust” methylation patterns towards those of normal biparental embryos, perhaps implying the existence of a dosage compensation mechanism similar to that of X-inactivation3. Possibly, therefore, such a process could influence the methylation patterns eventually observed in the androgenetic moles. Two examples of clonal analysis of AgCHM are shown. a, SNRPN. Figure 1c in the main text shows that both AgCHM displayed a minor degree of SNRPN DMR methylation (normally only seen on a maternal allele). The clones show a striking contrast to the strictly haplomethylated pattern we show above for H19 in the BiCHM. Our interpretation is that a degree of secondary methylation is occurring in AgCHM, but that it is incomplete and quite probably not allele-specific. The overall proportion of methylated C residues (14%) agrees with visual assessment of the directly sequenced PCR product. b, GNAS1-1A. Figure 2 in the main text indicates that one of the AgCHM showed approximately equal intensity of methylated and unmethylated signals at all the CpG positions. GNAS1-1A clones from this AgCHM are either almost completely methylated or unmethylated. It is unclear whether or not this reflects allele-specificity, since we cannot distinguish the two paternal alleles at this locus. It remains possible that the pattern results not from allele-specificity, but from cooperative kinetics of de novo methylation.
References
-
1.
Zeschnigk, M. et al. Imprinted segments in the human genome: different DNA methylation patterns in the Prader-Willi/Angelman syndrome region as determined by the genomic sequencing method. Hum Mol Genet 6, 387-95 (1997).
-
2.
Arima, T., Matsuda, T., Takagi, N. & Wake, N. Association of IGF2 and H19 imprinting with choriocarcinoma development. Cancer Genet Cytogenet 93, 39-47 (1997).
-
3.
Shemer, R. et al. Dynamic methylation adjustment and counting as part of imprinting mechanisms. Proc Natl Acad Sci USA 93, 6371-6 (1996).
Rights and permissions
About this article
Cite this article
Judson, H., Hayward, B., Sheridan, E. et al. A global disorder of imprinting in the human female germ line. Nature 416, 539–542 (2002). https://doi.org/10.1038/416539a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/416539a
This article is cited by
-
Reproductive Outcomes from Maternal Loss of Nlrp2 Are Not Improved by IVF or Embryo Transfer Consistent with Oocyte-Specific Defect
Reproductive Sciences (2021)
-
Pregnancy after oocyte donation in a patient with NLRP7 gene mutations and recurrent molar hydatidiform pregnancies
Journal of Assisted Reproduction and Genetics (2020)
-
A novel NLRP7 protein-truncating mutation associated with discordant and divergent p57 immunostaining in diploid biparental and triploid digynic moles
Virchows Archiv (2020)
-
Comprehensive analysis of 204 sporadic hydatidiform moles: revisiting risk factors and their correlations with the molar genotypes
Modern Pathology (2020)
-
A KHDC3L mutation resulting in recurrent hydatidiform mole causes genome-wide DNA methylation loss in oocytes and persistent imprinting defects post-fertilisation
Genome Medicine (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.