Article Text
Abstract
Gestational trophoblastic disease describes a group of rare pregnancy related disorders that span a spectrum of premalignant and malignant conditions. Hydatidiform mole (also termed molar pregnancy) is the most common form of this disease. Hydatidiform mole describes an abnormal conceptus containing two copies of the paternal genome, which is classified as partial when the maternal genome is present or complete when the maternal genome is absent. Hydatidiform mole typically presents in the first trimester with irregular vaginal bleeding and can be suspected on ultrasound but confirmation requires histopathological evaluation of the products of conception. Most molar pregnancies resolve without treatment after uterine evacuation, but occasionally the disease persists and develops into gestational trophoblastic neoplasia. Close monitoring of women after molar pregnancy, with regular measurement of human chorionic gonadotrophin concentrations, allows for early detection of malignancy. Given the rarity of the disease, clinical management and treatment is best provided in specialist centres where very high cure rates are achievable. This review looks at advances in the diagnosis and early management of gestational trophoblastic disease and highlights updates to disease classification and clinical guidelines. Use of molecular genotyping for improved diagnostic accuracy and risk stratification is reviewed and future biomarkers for the earlier detection of malignancy are considered.
- Pregnancy complications
- Pathology
- Medical oncology
- Biochemistry
- Genetics
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
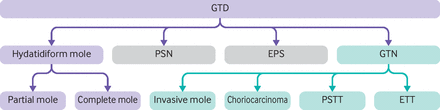
Gestational trophoblastic disease (GTD) describes a heterogeneous group of disorders that arise from abnormal proliferation of placental trophoblastic tissue (box 1). Diagnostic classification spans the pre-malignant conditions of complete hydatidiform mole (CHM) and partial hydatidiform mole (PHM) to the malignant conditions of invasive mole, choriocarcinoma, placental site trophoblastic tumour (PSTT) and epithelioid trophoblastic tumour (ETT), collectively known as gestational trophoblastic neoplasia (GTN) (figure 1). Both complete and partial moles have the potential for malignant transformation but the risk of GTN is higher for CHM (15-20%) than for PHM (0.5-1%)(table 1).1–3 Maternal age and history of a previous hydatidiform mole are two established risk factors for molar pregnancy. Hydatidiform moles are classified as complete or partial based on their morphology and genetic profile.
Glossary of abbreviations
GTD=gestational trophoblastic disease.
GTN=gestational trophoblastic neoplasia.
CHM=complete hydatidiform mole.
PHM=partial hydatidiform mole.
PSTT=placental site trophoblastic tumour.
ETT=epithelioid trophoblastic tumour.
Gestational trophoblastic disease (GTD) classification according to the WHO 2020 Classification of Female Genital Tumours.76 PSN=placental site nodule; EPS=exaggerated placental site reaction; GTN=gestational trophoblastic neoplasia; ETT=epithelioid trophoblastic tumour; PSTT=placental site trophoblastic tumour
Typical characteristics of complete and partial hydatidiform moles
Most cases of GTN occur after molar pregnancy but they can also occur after any gestational event, including miscarriage and ectopic or term pregnancies. GTN is the most curable of all gynaecological malignancies with cure rates approaching 100%, even in the presence of metastatic disease.3 The increased use of ancillary techniques has improved the accuracy of GTD classification and risk stratification based on morphology. This review focuses on the diagnosis and early management of GTD over the past decade and we direct the reader to other publications for expert opinion on the chemotherapeutic management of GTN.4–8
Sources and selection criteria
We searched PubMed, Medline, and CINAHL (EBSCO) for articles written in English and published from 1 January 2011 to 31 December 2021, using the search terms “gestational trophoblastic disease,” “hydatidiform mole,” “molar pregnancy,” and “gestational trophoblastic neoplasia.” We considered articles in peer reviewed journals and included articles based on study quality. We predefined the priority of study selection according to the level of evidence (clinical practice guidelines, systematic reviews and meta-analysis, cohort studies, and expert opinion). We consulted clinical management guidelines and expert reviews on this topic and included highly cited studies and those of historical significance. Relevant publications outside of the specified time were considered based on a review of bibliographies and expert opinion and the review bibliography was updated to 1 September 2022.
Genetic terminology
Diandric=two copies of the paternal genome.
Digynic=two copies of the maternal genome.
Monogynic=one copy of the maternal genome.
Androgenetic=paternal derived genome.
Endoreduplication=replication of the nuclear genome without mitotic cell division.
Diploidisation=conversion of a polyploid genome back into a diploid genome through a process of reduction of the duplicated genome.
Genomic imprinting=a mechanism of silencing genes by DNA methylation, resulting in gene expression that is specific to one parent.
Parental conflict hypothesis=a theory that imprinting provides a selective advantage in reproduction, such that placental specific genes are paternally imprinted and maternally expressed.
Incidence
International comparisons are difficult owing to the paucity of centralised registries worldwide, combined with an under-reporting of cases to registries, variation in case definition, and absence of a consensus based denominator.9–11 Incidence rates are typically derived from a mixture of live births, pregnancies, or deliveries but the total number of conceptions, although difficult to acquire, would be more inclusive.12
The incidence of hydatidiform mole varies worldwide with rates of 1-2 per 1000 pregnancies in Europe and North America compared with 10 per 1000 pregnancies in India and Indonesia.11 13 The incidence of GTD in the UK is one in 714 live births but incidence varies according to ethnic group, with the highest incidence reported in women of Asian descent.14–16 Genetic, social, cultural, and dietary factors might be relevant to GTD incidence. Of note, GTD frequency is higher in regions of the world where malnutrition is common.17 18 In the UK, the incidence of molar pregnancy is about one in 600 conceptions and the prevalence of PHM is higher than CHM at a ratio of 3:1.2 19 Prevalence of CHM is age dependent with higher frequencies at the lower and upper ends (<16 years and >40 years) of reproductive age.2 3
Choriocarcinoma is the most aggressive form of GTN with a reported incidence of one per 40 000 pregnancies in Europe and North America compared with 9.2 per 40 000 pregnancies in South East Asia. 11 ,20 In 2017, a systematic review of 121 case reports showed that 30% of choriocarcinomas had metastasised at the time of diagnosis.21 In April 2020, a larger UK based study of 234 cases reported metastasis in more than 50% of women with non-molar derived choriocarcinoma.22 Although most invasive moles originate from a CHM, only 25% of choriocarcinoma and 25% PSTT and ETT derive from a molar pregnancy.23 24 The risk of developing choriocarcinoma after a molar pregnancy is higher for CHM (2–3%) than PHM (<1%).25 26 Hence, gestational choriocarcinoma should be considered in all premenopausal women with metastases of an unknown primary.27
PSTT is a very rare form of GTN with an incidence of one in 100 000 deliveries in the UK, and ETT is even rarer.28–30 Owing to their rarity, PSTT and ETT are not discussed in detail in this review and readers are directed to other resources for details of their diagnosis and management.31–34
Clinical overview
Women with a molar pregnancy usually present with irregular vaginal bleeding in the first trimester. An ultrasound scan can identify most CHMs (88%) but only detects some PHMs (56%), presenting a diagnostic challenge.35 Although sonography might be suggestive of a molar pregnancy, histopathological examination of the products of conception is the gold standard for the diagnosis of molar pregnancy.36 In CHM, human chorionic gonadotrophin (hCG) might be inappropriately high and reach concentrations of more than 100 000 IU/L (table 1). A review of 180 cases presenting to the New England Trophoblastic Disease Centre over a 20 year period showed a reduction in the gestational age at diagnosis of CHM from 12 weeks to 9 weeks gestation.37 Earlier diagnosis of GTD has led to changes in clinical presentation with women now rarely presenting with anaemia, hyperemesis, pre-eclampsia, or hyperthyroidism.38 39
The diagnosis of GTN is largely based on a combination of obstetric history and elevated concentrations of hCG.40 After molar pregnancy, plateaued or rising hCG concentrations are indicative of GTN. All forms of GTN (excluding PSTT and ETT) are highly vascular and a biopsy is not recommended because of the risk of a life threatening haemorrhage. Identification of the origin of choriocarcinoma can be challenging and genetic profiling can help to differentiate gestational from non-gestational choriocarcinoma, with non-gestational choriocarcinoma having a worse prognosis.
Obstetric management of molar pregnancy involves uterine evacuation and histopathological examination of the products of conception. Follow-up serum or urine hCG monitoring is done until hCG values return to within the normal range. hCG is an ideal biomarker for GTD surveillance as its concentration accurately reflects disease burden. Most women with molar pregnancy do not require further treatment following uterine evacuation of the products of conception. However, some women develop disease persistence and progress to malignant disease requiring chemotherapy or further surgical intervention.
Twin pregnancy
A twin pregnancy of a CHM with a coexisting viable fetus is rare, occurring in one in 20 000-100 000 pregnancies.41 In these cases, the most common combination is a CHM and normal fetus.42 A UK study of 77 twin CHM pregnancies reported a live birth rate of 40% and found that the risk of GTN did not increase beyond the first trimester, obviating the need for early termination of pregnancy.41 A subsequent restrospective cohort study of 75 twin CHM pregnancies at the same centre reported even higher live birth rates (51%). This study also reported a higher malignancy risk in CHM twin (20%) pregnancies than in CHM (16%) pregnancies without a co-twin.43 Another larger multicentre cohort study reported a slightly higher malignancy risk (27%) in CHM twin pregnancies.44 45 A systematic review of obstetric outcomes in CHM twin pregnancies showed a higher risk of perinatal complications and low live birth weight with a third of the women progressing to GTN.46 Multidisciplinary management of these pregnancies in specialist centres is recommended to ensure early detection of pregnancy complications.14
Risk factors for the development of gestational trophoblastic disease
Potential risk factors for the development of molar pregnancy include ethnicity, maternal age, and history of a hydatidiform mole.19 47 48 As previously mentioned, molar pregnancy is more prevalent in women at the lower and upper ends of reproductive age (<16 and >40 years). Adolescents (ages 13-16 years) have a slightly increased risk (1:450) of molar pregnancy, which increases to 1:157 (40 years of age), and rises sharply to 1:8 (≥50 years of age).2 9 47 Most molar pregnancies are sporadic but a history of hydatidiform mole increases the risk of a subsequent mole, which is often the same type as the index mole.49–51 The risk of a second molar pregnancy is about 1% and this risk is greater for CHM than PHM. The risk increases to 15-20% after two hydatidiform moles.4 50 Of note, the risk is independent of the male partner, suggesting an underlying defect in oocyte function.52 Supporting this concept, some recurrent hydatidiform moles have an inherited methylation defect in a gene (NLRP7) associated with oocyte maturation and placental development.53 54 Evidence of oocyte defects in other species has also been reported. Female mice with an inherited meiotic abnormality in their oocytes (MEI1) produce androgenetic zygotes by extruding all maternal chromosomes and their spindles into the first polar body, and a similar mechanism could exist in humans.55
Recent evidence suggests that women who have early diagnosis of PHM as their first gestational event are more likely to develop postmolar GTN.56 Studies have postulated a link in some countries between deficiency of carotene (vitamin A precursor) and a higher incidence of CHM.57 58Rodent studies have also shown that diet can reset the genetic imprint.59 Deficiency of vitamin A or folates in early gestation (18-21 days) is associated with the absence of placental villous vascularity, as seen in CHM.60 Hispanic women have a substantially lower risk of developing GTN after CHM than white women, suggesting a protective role for environmental or genetic factors.61
Genetic classification of a hydatidiform mole
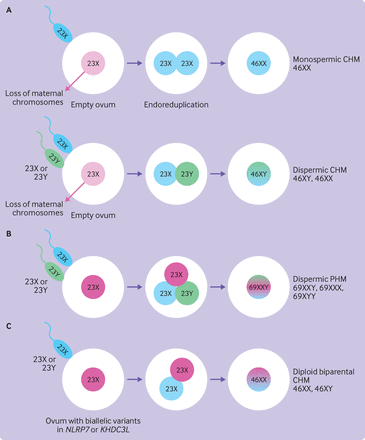
The genetic origin of hydatidiform mole is complex (figure 2). CHMs have a purely androgenetic genome. They contain 46 chromosomes (diploid) with two copies of the paternal genome (diandric) and no contribution from the maternal genome (box 2). Heterozygous dispermic CHMs are clinically more aggressive than homozygous monospermic CHM and have a significantly higher risk of neoplastic transformation.62 These moles result in higher mean hCG concentrations and a threefold increased risk of progression to GTN.62–64 Rare cases of tetraploid CHM also exist and require the same close surveillance as diploid CHM.65 PHMs have 69 chromosomes (triploid) and contain two paternal (diandric) and one maternal (monogynic) genome. Rare triandric tetraploid PHMs have been reported, which appear to arise from fertilisation of an ovum by three different sperm.66
Genetic origin of hydatidiform moles. (A) CHMs have a diploid genome. Most CHMs (80-90%) arise from fertilisation of an empty ovum by a haploid sperm, which undergoes endoreduplication to result in diploidy (homozygous monospermy). Some CHMs (10-20%) result from fertilisation of the empty ovum by two sperm resulting in diploidy (heterozygous dispermy). (B) PHMs have a triploid genome. Most PHMs (>95%) arise from fertilisation of a single oocyte by two different sperm (heterozygous dispermic). (C) Familial recurrent hydatidiform moles due to biallelic variants in maternal effect genes (NLRP7 or KHDC3L) result in biparental recurrent CHMs. CHM=complete hydatidiform mole; PHM=partial hydatidiform mole
Familial recurrent hydatidiform mole
Familial recurrent hydatidiform mole is a rare autosomal recessive disorder associated with a predisposition to molar pregnancy.67 These recurrent CHMs have a diploid biparental origin and the inheritance of biallelic variants in maternal effect genes (NLRP7 or KHDC3L) disrupts genomic imprinting.68 69 These genes are normally expressed in the oocyte and support embryonic development until gene expression in the embryo becomes active.68 In Mexico, a study of 44 unrelated women with at least two hyatidiform moles suggested a founder effect in NLRP7 (L750V) with most cases occurring in consanguineous families.70 Familial recurrent CHM should be suspected in women presenting with two CHMs and genetic analysis should be requested to determine recurrence risk.71 Women with familial recurrent hydatidiform mole usually require in vitro fertilisation with ovum donation to help them have a normal pregnancy.53 Notably, a UK study of 166 women with at least two molar pregnancies found that one in 640 women registered with a complete mole had familial recurrent CHM.50
Origin of hydatidiform mole formation
Genomic imprinting is a term used to describe the parental specific expression of certain genes. Some genes are imprinted (suppressed) when paternally inherited and expressed when maternally inherited and vice versa. The parental conflict hypothesis was proposed to explain genomic imprinting in the placenta, whereby placental specific genes are paternally imprinted and maternally expressed.72 Consistent with this hypothesis, the lack of a maternal genome and over-representation of the paternal genome in CHM results in impaired genomic imprinting and proliferation of the villous trophoblast.73
Several theories have emerged to explain the origin of hydatidiform moles. One theory is that complete moles originate from an empty ovum, which is then fertilised by one or two sperm (figure 2).74 However, the finding of rare CHMs with retained maternal chromosome 11 suggests that the ovum is not completely devoid of all maternal DNA.73 An alternative theory proposed by Golubovsky postulates that all hydatidiform moles originate from the fertilisation of a normal ovum by two sperm to create a triploid conceptus. A complex postzygotic event then excludes the maternal genome and results in diploidisation to create a CHM.74 75
Pathological classification
According to the WHO 2020 Classification of Female Genital Tumours, GTD can be subdivided into molar pregnancies or hydatidiform moles, GTN, tumour-like (non-neoplastic) lesions, and abnormal (non-molar) villous lesions.76 The pathogenesis of GTD is unique as the maternal tumour arises from gestational tissue rather than maternal tissue.77 Histopathological classification of products of conception into CHM, PHM, and non-molar gestations can be challenging based on morphology alone, and ancillary techniques (eg, immunohistochemistry, ploidy analysis, and molecular genotyping) are used to aid diagnosis.
Placental site nodules and exaggerated placental site reactions are classified as benign tumour-like trophoblastic lesions. These lesions usually present as incidental findings in tissue after hysterectomy or endometrial biopsy. Placental site nodules are gestational tissue remnants left in the uterine wall that do not fully regress and disappear after a normal pregnancy.40 Larger placental site nodule-like lesions with atypical histopathological features are described as atypical placental site nodules. A retrospective review of atypical placental site nodules in one centre in the UK recorded neoplastic transformation in three (14%) of 21 cases within 16 months of diagnosis.78 As a result, women with atypical placental site nodules are registered with GTD centres, have central pathology review, hCG monitoring, and are offered a hysterectomy if deemed appropriate.40
Choriocarcinoma can be gestational or non-gestational and both have different routes of metastasis and treatment regimens. Non-gestational choriocarcinoma can be somatic or derived from the germ cell and has a high propensity for metastasis.79 Use of molecular genotyping to confirm the absence of paternal DNA can lead to an accurate classification of non-gestational choriocarcinomas.80
The diagnosis of CHM, PHM, placental site nodule, and atypical placental site nodule is made on the basis of histopathological confirmation.14 In contrast, diagnosis of GTN does not usually rely on histopathological confirmation as biopsies are not always available. The treatment of invasive mole and choriocarcinoma is often initiated on the basis of a rising hCG level even in the absence of other clinical evidence of disease recurrence.81
Pathological diagnosis
Earlier clinical diagnosis of GTD is now possible due to improved ultrasound sensitivity but it often makes pathological differentiation of early CHM and PHM from non-molar gestations more challenging. The features of a mole are more subtle at early gestation and non-molar pregnancies, which often feature genetic abnormalities, can show morphological appearances that overlap to varying degrees. As a result, ancillary techniques are often required to assist morphological diagnosis.
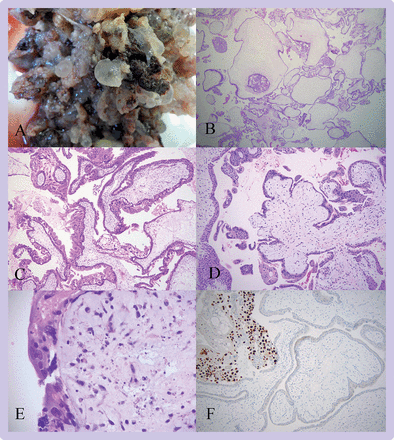
Morphologically, the classical appearance of CHM is characterised by the presence of a diffuse population of enlarged, hydropic villi with cistern formation. Fetal tissue is generally absent. Villi are irregular with formation of trophoblast pseudoinclusions. Non-polar or circumferential proliferation of villous trophoblast is prominent and the included extravillous trophoblast shows cytological atypia (figure 3). In CHM diagnosed in the first trimester, villous enlargement and trophoblast proliferation can be less developed and diagnosis is more challenging. In these cases; useful diagnostic features include the presence of villi with bulbous, cauliflower-like or knuckle-like outlines. These villi often have abnormal dense, blueish, myxoid stroma with prominent karyorrhectic nuclear debris.73 82
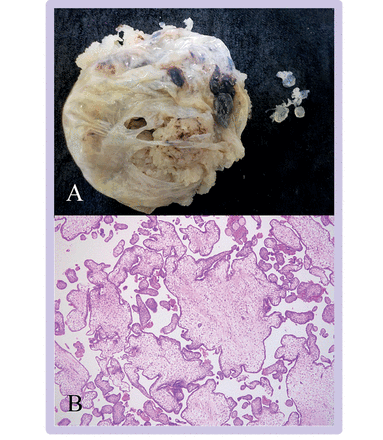
Complete hydatidiform mole. (A) In this macroscopic image of a product of conception, extensive formation of vesicles is evident giving a bunch of grapes effect. (B) The villous population is diffusely abnormal with numerous enlarged hydropic villi (16X). (C) The villi in this view are highly irregular and most show non-polar or circumferential trophoblast hyperplasia (50X). (D) In this early complete hydatidiform mole, the central villous shows the typical cauliflower-like outline with bulbous, knuckle-like projections (50X). (E) On higher magnification this early complete hydatidiform mole has abnormal, dense, myxoid stroma with prominent stromal karyorrhexis (400X). (F) p57 immunohistochemistry shows loss of staining in villous cytotrophoblast and stromal cells with preservation of staining in extravillous trophoblast (upper left) (100X)
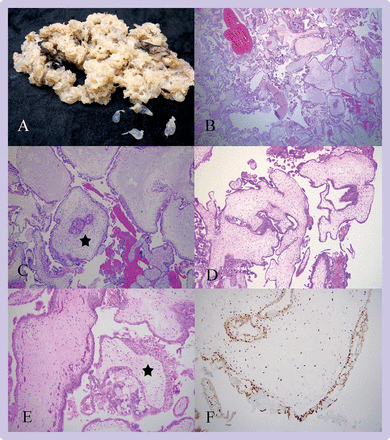
In contrast to CHM, PHM is characterised by a mixed population of enlarged hydropic villi with some cisterns and small, more normal appearing or fibriotic villi. An abnormal fetus can be present. Villous outlines are irregular and scalloped with fjord-like indentations and formation of trophoblast pseudoinclusions (figure 4). Villous trophoblast abnormalities, in contrast to CHM, are less prominent with focal non-polar or circumferential trophoblast hyperplasia.73 82
Partial hydatidiform mole (PHM). (A) In this macroscopic image of a PHM, focal vesicle formation is evident with some vesicles separating from the specimen at bottom right. (B) This low power magnification shows a mixture of villous types with a group of hydropic villi seen on the right and smaller, more normal sized villi on the left (16X). (C)The villus with a star has a trophoblast pseudoinclusion (50X). (D) The PHM villi are markedly irregular with fjord-like indentations (50X). (E) Focal non-polar trophoblast hyperplasia is affecting the villus with the star while the surrounding villi show little or no hyperplasia (100X). (F) p57 immunohistochemistry is normal (100X)
Mimics of molar pregnancy show one or more of the aforementioned features and thus need to be distinguished from a hydatidiform mole based on their more limited morphological features of a mole or use of ancillary techniques. Hydropic abortions are characterised by a diffuse population of hydropic villi but they are generally regular in outline and show no significant trophoblast hyperplasia. Non-molar products of conception that show morphological abnormalities, termed abnormal villous morphology, can mimic molar pregnancy and can have some of the features of a mole, such as small trophoblast pseudoinclusions, irregular villous outlines, or focal abnormal villous trophoblast hyperplasia. Some specimens with abnormal villous morphology can result from genetic abnormalities, such as aneuploidy (figure 5).83
Abnormal villous morphology. (A) This macroscopic image of a second trimester placenta has a number of clear vesicles becoming detached from the specimen. (B) On microscopy of this placenta,villous morphology was abnormal with villous enlargement, some irregularity in villous outlines, and small pseudoinclusions (50X). This case was confirmed on microarray of fresh tissue to be an example of mosaicism for monosomy X (Turner syndrome)
Immunohistochemistry and ploidy analysis using cytogenetics, flow cytometry, or fluorescent in situ hybridisation and molecular genotyping can help to distinguish moles from mimics and improve the accuracy of a GTD diagnosis. Use of cytogenetics for ploidy analysis can also identify aneuploidies, which can help to explain a particular pregnancy loss.
p57 immunohistochemistry
The protein p57kip2 (also termed p57) is a cyclin dependent kinase inhibitor encoded by the gene CDKN1C on chromosome 11p15.5. This gene is paternally imprinted and maternally expressed. When the maternal genome is present (in non-molar products of conception or PHM), p57 is expressed in placental villi and stromal cells and cytotrophoblast stain positively. In complete moles (diploid diandric), p57 is not expressed in cytotrophoblast and villous stroma and staining in these cells is lost. Staining is, however, preserved in the decidua and extravillous trophoblast, which act as internal controls for p57 immunohistochemistry.58 As a readily available test, p57 immunostaining can support the morphological diagnosis of CHM in routine practice.
Rarely, other challenges in the interpretation of p57 immunohistochemistry arise (eg, when a maternal chromosome 11 is retained in a CHM and p57 continues to be expressed, or if a maternal chromosome 11 is lost in a PHM and p57 immunostaining is lost).82 84 Further ancillary testing and close correlation with morphology is required to prevent misdiagnosis in these cases.
One category of morphologically abnormal products of conception in the differential diagnosis of molar pregnancy results from androgenetic/biparental mosaicism, which can be recognised by their unusual patterns of p57 staining.85 86 In p57 discordant villi, the villous stromal cells are p57 negative but the cytotrophoblast are p57 positive (figure 6). Inverted p57 discordant villi have the reverse pattern where the cytotrophoblast are negative and stromal cells are positive. Staining can also be divergent where two or more populations of villi exist with different staining patterns.85
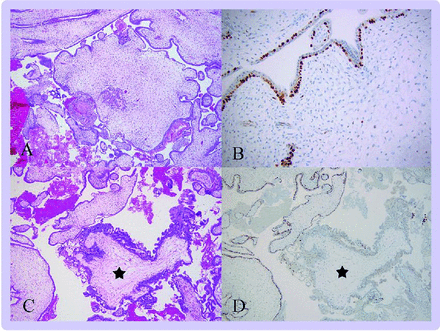
p57 discordant villi. (A)This low power magnification shows abnormal villous morphology with enlarged, hypercellular villi and a trophoblast pseudoinclusion but no trophoblast hyperplasia (50X). (B) Immunohistochemistry for p57 shows a discordant pattern with positive cytotrophoblast and negative stromal cells (100X). (C) Careful examination of the specimen identified a small focus of villi with trophoblast hyperplasia (star) (50X). (D) This focus of villi showed absence of p57 staining in stroma and cytotrophoblast in keeping with a component of CHM (50X).
Although uncommon, these types of products of conception are important because they might harbour a component villous population that is purely androgenetic and therefore represents a CHM and thus requires the appropriate clinical follow-up.85 In addition to their representation in early gestation products of conception, these patterns can be seen in the second and third trimester in placental mesenchymal dysplasia (p57 discordant villi)87 88 and as a focal finding in otherwise normal placentas (inverted p57 discordant villi).89
In general, p57 assists in the differential diagnosis of CHM, and ploidy analysis is frequently used to support morphology and confirm or exclude a triploid conceptus in the differential diagnosis of PHM. However, ploidy will not distinguish between triploidy with two paternal (diandric) or two maternal (digynic) contributions to the genome and thus must be used in combination with morphological assessment.
Molecular genotyping
Molecular genotyping is considered the gold standard for hydatidiform mole classification because this process can establish ploidy and identify the parental origin of the molar tissue. Molecular genotyping can also help to differentiate complete and partial moles from GTD mimics. Analysis of polymorphic short tandem repeat DNA sequences on multiple chromosomes in the human genome is used to determine genotype.24 The short tandem repeat profiles in the placental villi of molar tissue are compared with those obtained from the maternal DNA in decidua to classify the mole. The clinical accuracy of short tandem repeat genotyping to refine the diagnosis of GTD has been shown by various studies.90 91 Some pitfalls in the use of molecular genotyping include the analysis of familial recurrent hydatidiform moles, which have a diploid biparental genome and can be misinterpreted as a non-molar gestation. In addition, an egg donor pregnancy, which does not carry alleles from the recipient mother, can be misinterpreted as a diandric complete mole. Close correlation of morphology, p57 immunostaining, and genotyping is required to ensure the correct diagnosis is reached in all cases.64
Biochemical diagnosis
hCG is a member of the glycoprotein hormone family that includes three pituitary hormones: luteinising hormone, follicle stimulating hormone, and thyroid stimulating hormone. These hormones are heterodimers that share a common alpha (α) subunit but have a unique hormone specific beta (β) subunit. hCG is a heterogenous molecule with many different isoforms produced by complex post-translational modification. In early pregnancy (three weeks gestation), hyperglycosylated hCG is produced by the syncytiotrophoblasts of the placenta to promote growth and differentiation.92 The main hCG variants found in serum or urine include intact hCG (α and β) along with fragments of nicked hCG, nicked hCGβ, and hCGβ core fragment.93 These five biologically active hCG isoforms are clinically relevant in gestational trophoblastic disease.94
Human chorionic gonadotrophin assays
Most commercial immunoassays were developed for the detection of hCG in early pregnancy. Total hCG assays detect intact and free β-subunit but will not necessarily detect all isoforms secreted in GTD.95 96 There is no international standardised hCG assay approved for use in women with GTD. Ideally hCG should be monitored with an assay that detects all hCG isoforms in equimolar amounts.97 98 GTD reference centres should have access to at least two hCG assays, one for primary analysis and another for confirmatory diagnosis. Several factors contribute to the variability in hCG assays including assay calibration, analyte specificity, and antibody heterogeneity. In the absence of centralised hCG monitoring, hCG should be measured using the same assay and analytical platform throughout follow-up to avoid inter-assay variability.96
In molar pregnancy, hCG is monitored postoperatively until normalisation is achieved. However, no consensus exists on what constitutes as normalisation because this term is assay dependent and can depend on the functional sensitivity of the assay and confidence interval used to define normality. Thus, the surveillance period can be prolonged in women monitored in a centre using a normal reference range of less than 1 IU/L as opposed to the commonly quoted reference range of less than 5 IU/L.
Immunoassay interference
All tumour markers measured by immunoassay are subject to false positive and false negative results from analytical or biological interference. Analytical interference in serum due to the presence of heterophilic antibodies (eg, anti-mouse antibodies) usually produces false positive results. A paired urine hCG measurement will not be subject to this interference as heterophilic antibodies are retained in the kidney. Non-linear dilution of serum samples might also suggest antibody interference.99 Heterophilic blocking tubes or polyethylene glycol precipitation can be used to remove antibody interference in serum.100 101 Another cause of erroneous results in immunoassay is due to a phenomenon known as the high dose hook effect which occurs when very high concentrations of hCG (>500 000 IU/L) generate a falsely low result.102 This effect occurs when excess analyte (hCG) saturates the assay antibodies and results in underestimation of analyte concentration. Dilution of the patient’s serum will bring the analyte concentration back into the measuring range of the assay and allow accurate quantitation. Clinicians and scientists need to be aware of the limitations of their hCG assay and ensure that suspicious results not fitting the clinical picture are discussed. Further investigations could involve use of an alternate assay or sending the sample for reanalysis to a GTD reference centre.
Persistent low level hCG elevations
Persistent low level hCG elevations can indicate disease recurrence or lack of response to treatment. In addition, some women with quiescent GTD have persistently low levels of hCG without clinical or radiological evidence of disease.103 Diagnostic interpretation can also be complicated by a pregnancy or the presence of pituitary derived-hCG in postmenopausal women. hCG concentrations can still be within the normal range when elevated up to 14 IU/L in women aged 55 years or older.104 Snyder et al provide an algorithm for investigating pituitary derived hCG and the European Organisation of Trophoblastic Disease practical clinical guidelines suggest further ways to investigate persistent low level hCG elevations.105 106 Familial hCG syndrome, a rare inherited form of persistently elevated non-functional hCG, can also complicate hCG monitoring.106
Inaccurate serum hCG results can have serious adverse consequences for patient management. Cole et al reported a series of 12 women diagnosed with gestational choriocarcinoma due to false positive hCG results.107 Seven of these women had either major surgery or chemotherapy and five had procedures which resulted in loss of fertility. Concurrent testing of serum and urine hCG in a GTD reference centre resolved all cases with false positives results due to circulating heterophilic antibodies.107
Clinical guidelines
In the absence of randomised controlled trial data for this rare disease, systematic reviews and consensus expert opinion have informed the development of international clinical GTD guidelines.108 109 Clinical practice guidelines have been generated by a number of organisations including the Royal College of Obstetrics and Gynaecologists, European Society for Medical Oncology, and European Organisation of Trophoblastic Disease.4 5 14
The European Organisation of Trophoblastic Disease clinical working party published practical clinical guidelines for GTD in 2020 in an effort to harmonise practice across Europe.5 This guideline advises when women should be referred to specialist GTD centres where expert advice is available. They recommend a centralised model of care with central hCG monitoring and pathology review because a large retrospective observational study showed that centralised pathology review altered the diagnosis in 40% of cases.110 Management of women with GTD by physicians who are experienced in trophoblastic disease has also resulted in better survival outcomes than when they are treated outside trophoblastic reference centres.111 112
An update to the Royal College of Obstetrics and Gynaecologists clinical guidelines for GTD in 2021 recommends registration of all affected women with a GTD centre as a minimum standard of care.14 The Irish clinical guidelines endorse this recommendation and advise centralisation of hCG measurement in a quality assured laboratory using an oncology approved assay.113 All guidelines recommend hCG monitoring of women after molar pregnancy to enable early detection of disease persistence.
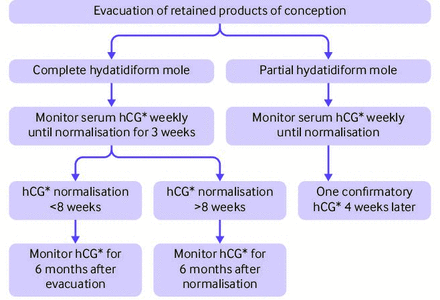
A large retrospective UK study of 20 000 women who had hCG monitoring after a molar pregnancy found the risk of GTN after hCG normalisation was 0.25% for CHM and 0.03% for PHM.114 A systematic review of 19 independent studies reported a slightly higher risk of GTN (0.35%) after hCG normalisation in CHM.115 Another study found that prolonged hCG surveillance particularly after PHM is not cost-effective given the rarity of GTN.116 Consequently, the Royal College of Obstetrics and Gynaecologists guidelines recommend a shorter hCG surveillance period after PHM with monitoring complete after two normal hCG values are obtained, one month apart. Complete moles require a longer surveillance period reflecting their higher risk of GTN. In CHM cases where hCG normalisation occurs within 56 days (eight weeks), women have hCG monitoring for a total of six months after uterine evacuation. However, when hCG normalisation occurs beyond 56 days, women require hCG monitoring for six months after normalisation (figure 7).14 113 114 Another update in the Royal College of Obstetrics and Gynaecologists guideline concerns women who did not require chemotherapy after molar pregnancy. These women no longer require a hCG test or histopathological examination of their placental tissue after future normal pregnancies.117
hCG monitoring protocol for complete and partial hydatidiform moles. hCG=human chorionic gonadotrophin.*hCG monitoring is done on the same analytical platform throughout follow-up. Adapted from the Royal College of Obstetrics and Gynaecology guidelines.14 Reproduced with permission Joyce and colleagues143
Women with invasive mole or choriocarcinoma are stratified into low or high risk GTN categories based on the Federation of Gynaecology and Obstetrics (FIGO) staging and modified WHO prognostic scoring system (tables 2 and 3).
Federation of Gynaecology and Obstetrics (FIGO) staging for GTN
World Health Organization modified prognostic scoring system for GTN
The FIGO scoring system is endorsed by all international GTD guidelines and is based on three main measures: post-evacuation hCG concentration, presence of metastatic disease, and histopathological diagnosis. A doppler pelvic ultrasound should be performed to confirm the absence of a pregnancy and ascertain the size of any intrauterine tumour. Chest x ray as opposed to computed tomography is the preferred imaging modality for detection of pulmonary metastases.4 Some centres consider a hCG concentration of 20 000 IU/L or more, four weeks after uterine evacuation as an indication for immediate chemotherapy but this recommendation has not been adopted by FIGO.118 Genomics might also be incorporated into future updates to the FIGO scoring system to reflect the critical role of molecular genotyping in identifying the antecedent pregnancy for GTN.
Women with low risk disease (score of ≤6) without metastatic disease are offered single drug chemotherapy or hysterectomy. Whereas women with high risk disease (score of ≥7) are offered multidrug chemotherapy, ideally under the supervision of an expert in GTD management. In the event of drug resistance or disease related complications, surgery can be considered. Women with ultra-high risk disease (score of >12) generally present with liver or brain metastases and require specialist multidisciplinary care. An adjustment to the FIGO scoring system is required to identify women at low risk who become resistant to single drug chemotherapy to enable them be treated at the outset with multidrug chemotherapy.7
Women treated with chemotherapy after a molar pregnancy are advised to avoid pregnancy for at least a year when the risk of relapse is greatest (3%) and an increase in hCG concentration might prevent early detection of disease recurrence.119 Advice on safe contraception after a molar pregnancy can be found in national fertility guidelines.120 A systematic review found no evidence for an association between oral contraceptive use during follow-up after a mole and the incidence of GTN.121 Moreover, a Brazilian retrospective cohort study found no association between hormonal contraceptive use during molar pregnancy follow-up or GTN treatment and the risk or severity of GTN, nor did it postpone the normalisation of hCG concentrations.122 In a retrospective review of 1532 women with gestational trophoblastic tumours who were treated with chemotherapy, 230 became pregnant within 12 months of finishing chemotherapy and five of these women relapsed. However, the relapse rate in women following chemotherapy was not higher in those women who became pregnant within the first year. This study also reported that single drug chemotherapy had no effect on fetal outcomes but that multidrug chemotherapy might have a transient effect on fertility and increase the risk of miscarriage.123
Importantly, all guidelines recommend that GTN should be considered in the differential diagnosis of all women who present with irregular vaginal bleeding after pregnancy and that serum hCG measurement should be included in the diagnostic investigations.39
Advances in diagnostics and therapeutics
Molecular genotyping has a central role in establishing the genomic origin of trophoblastic tumours and informs prognosis and treatment options. When tumour tissue is inaccessible, liquid biopsy can provide a non-invasive method of analysing circulating tumour DNA in maternal blood to confirm the genetic origin of choriocarcinoma.124 In a study of 20 women with GTN, short tandem repeat analysis of circulating tumour DNA provided a genetic diagnosis in all but three cases. Women without a diagnosis had low levels of circulating tumour DNA and concurrent low hCG levels reflecting a low tumour burden. Digital droplet polymerase chain reaction combined with single nucleotide polymorphism analysis can provide a more sensitive diagnostic technique for such cases.125
The use of circulating gestational trophoblasts to establish the genetic origin of trophoblastic disease shows promise. A comparative study found circulating gestational trophoblasts superior to circulating tumour DNA for confirming a diploid androgenetic conceptus.126 Use of circulating gestational trophoblasts has the advantage of using single cells to allow better discrimination of mosaicism and is not subject to maternal DNA interference, which simplifies result interpretation.
Matrix metalloproteinases, which facilitate extra-cellular matrix degradation, might also allow malignant trophoblast cells invade the maternal uterus.127 The high expression of matrix metalloproteinases and low expression of their inhibitors in choriocarcinoma might explain its invasiveness and malignant potential.128 129 Similarly, the wingless signalling pathway (Wnt gene family), which regulates placental cell migration, might be implicated in trophoblastic disease. Methylation based silencing of Wnt signalling inhibitors might enable Wnt hyperactivation and facilitate trophoblast invasion reported in cases of CHM and choriocarcinoma.130 Therefore, drugs that inhibit matrix metalloproteinase or downregulate Wnt signalling might provide useful future therapeutic options.
High concentrations of the angiogenic factor BMP-9 have been associated with chemoresistance to primary methotrexate therapy. The combined use of serum BMP-9 levels with an ultrasound biomarker for tumour vascularity (uterine pulsatile index) shows promise in helping to predict which women will develop methotrexate resistance.131 Furthermore, algorithms that use hCG regression nomograms to predict early chemoresistance might prove useful for clinical managment but they are assay specific and not widely adopted.132
Use of checkpoint inhibitor immunotherapy (eg, pembrolizumab) for chemoresistant, very high risk GTN has had some success.133 Immunotherapy targets the T cell receptor, PD-L1, which is highly expressed on normal trophoblasts and all forms of GTN.7 133 The presence of tumour infiltrating lymphocytes can serve as a biomarker to select women who might respond to pembrolizumab.53 Another PD-L1 inhibitor, avelumab, was found to be safe and effective in GTN cases resistant to single drug chemotherapy.134 An alternative salvage treatment for chemoresistant GTN involves use of camrelizumab plus apatinib.135 Hence, clinical trials are evaluating the use of checkpoint inhibitors alone or in combination with chemotherapy to target choriocarcinoma.108
Women with low risk GTN (FIGO score of 5-6) who have a high chance of resistance to first line therapy (methotrexate or actinomycin-D) could be risk stratified to combination therapy based on prognostic factors (hCG concentration before treatment, metastatic disease status, and choriocarcinoma histopathology).136 In particular, women with methotrexate resistance could be treated with ATR or CDK4/6 inhibitors.137
Effect of diagnosis on women and their families
Despite excellent cure rates, the psychosocial consequences of GTD are complex and clinicians need to be mindful of the need for counselling and psychological support for these women. Cancer specific distress, future fertility fears, mood, and sexual disturbances can persist for years in affected women.129 138 A systematic review of health related quality of life outcomes in women with GTD found substantial levels of anxiety, depression, sexual dysfunction, and fertility related distress, especially in women treated for GTN.139 In a prospective study published in 2022 of the psychological impact of GTD on 60 women, 47% reported feeling anxious and 70% reported feeling distressed during the surveillance period.140 Unfortunately, this study did not record previous mental health status or antidepressant use of the participants which was highlighted in a follow-up letter to the editor.141 142 A survey of women on the Irish national GTD registry found that women experienced feelings of intense sadness at the time of diagnosis and needed psychological support to help them to deal with the pregnancy loss. The need for information leaflets, psychological counselling, bereavement care guidance, and peer support was greatest in the first year after diagnosis.143 A fear of disease recurrence and concerns about future pregnancies accounted for some of the psychological distress experienced by these women.139 144 145 Healthcare professionals treating affected women need to be mindful of the psychological wellbeing of women and their partners as a consequence of GTD and they need to be alert to their desire for supportive services.
Conclusion
The prognosis for women after a molar pregnancy is excellent but some uncertainty remains around the cause of GTD, the risk factors that contribute to malignant transformation, and the optimum surveillance period. The increased use of molecular genotyping has improved the diagnostic accuracy of GTD classification, which is critical for prognostic stratification. Further work is needed to standardise hCG assays and identify those assays that are most appropriate for use in oncology. At this time, no effective prognostic biomarker is available to specifically identify those few women who will develop malignancy after molar pregnancy and require chemotherapy.
The treatment options for GTN over the past decade have improved considerably with most women now cured and salvage treatment pathways available for those who develop chemoresistance. As our understanding of GTD evolves, we might identify more sensitive biomarkers to detect disease progression earlier and to reduce the lengthy surveillance period, which impacts future pregnancy planning. Identification of women with GTD is of paramount importance because this disorder is highly curable.
Questions for future research
How do we define human chorionic gonadotrophin normalisation?
What is the optimum surveillance period after molar pregnancy?
How can we predict which women will develop malignancy after molar pregnancy?
What is the genomic imprinting defect in hydatidiform mole?
How can we improve the reporting and surveillance of gestational trophoblastic disease to ensure more accurate incidence rates?
Patient involvement
When planning this review, the opinion of a patient diagnosed with a complete hydatidiform mole was sought. This woman developed persistent disease after a molar pregnancy and required multidrug chemotherapy. She was provided with a draft outline of the review and based on her feedback, a section on the psychological impact of gestational trophoblastic disease diagnosis on women and their families was included in the review.
Acknowledgments
We wish to thank our patient representative, Evelyn Kingston Mythen, for her valuable contribution to this review.
References
Footnotes
Twitter @carolinemjoyce, @keelinodonoghue
Contributors CMJ developed the review outline, performed the literature review, and wrote all sections of the manuscript. KOD developed the review outline, reviewed the manuscript, and is supervising CMJ for her PhD. BF provided the pathology images and critically reviewed the manuscript. TMC reviewed and edited the manuscript and is supervising CMJ for her PhD. JC reviewed and edited the manuscript. All authors approved the final version of the manuscript. KOD is the guarantor.
Funding CMJ is funded by the Irish Research Council under grant number EBPPG/2021/38.
Competing interests We have read and understood the BMJ Medicine policy on declaration of interests and declare the following interests: none.
Provenance and peer review Commissioned; externally peer reviewed.